1. Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J Dent Res. 2005; 84:9–20. PMID:
15615869.

2. Hara AT, Livengood SV, Lippert F, Eckert GJ, Ungar PS. Dental surface texture characterization based on erosive tooth wear processes. J Dent Res. 2016; 95:537–542. PMID:
26848070.

3. Merijohn GK. Management and prevention of gingival recession. Periodontol 2000. 2016; 71:228–242. PMID:
27045439.

4. Solís Moreno C, Santos A, Nart J, Levi P, Velásquez A, Sanz Moliner J. Evaluation of root surface microtopography following the use of four instrumentation systems by confocal microscopy and scanning electron microscopy: an
in vitro study. J Periodontal Res. 2012; 47:608–615. PMID:
22494068.
5. Amid R, Kadkhodazadeh M, Fekrazad R, Hajizadeh F, Ghafoori A. Comparison of the effect of hand instruments, an ultrasonic scaler, and an erbium-doped yttrium aluminium garnet laser on root surface roughness of teeth with periodontitis: a profilometer study. J Periodontal Implant Sci. 2013; 43:101–105. PMID:
23678394.

6. Santos FA, Pochapski MT, Leal PC, Gimenes-Sakima PP, Marcantonio E Jr. Comparative study on the effect of ultrasonic instruments on the root surface in vivo
. Clin Oral Investig. 2008; 12:143–150.
7. Lee HJ, Lee J, Lee JT, Hong JS, Lim BS, Park HJ, et al. Microgrooves on titanium surface affect peri-implant cell adhesion and soft tissue sealing; an
in vitro and
in vivo study. J Periodontal Implant Sci. 2015; 45:120–126. PMID:
26131372.
8. Lee HJ, Yang IH, Kim SK, Yeo IS, Kwon TK.
In vivo comparison between the effects of chemically modified hydrophilic and anodically oxidized titanium surfaces on initial bone healing. J Periodontal Implant Sci. 2015; 45:94–100. PMID:
26131369.
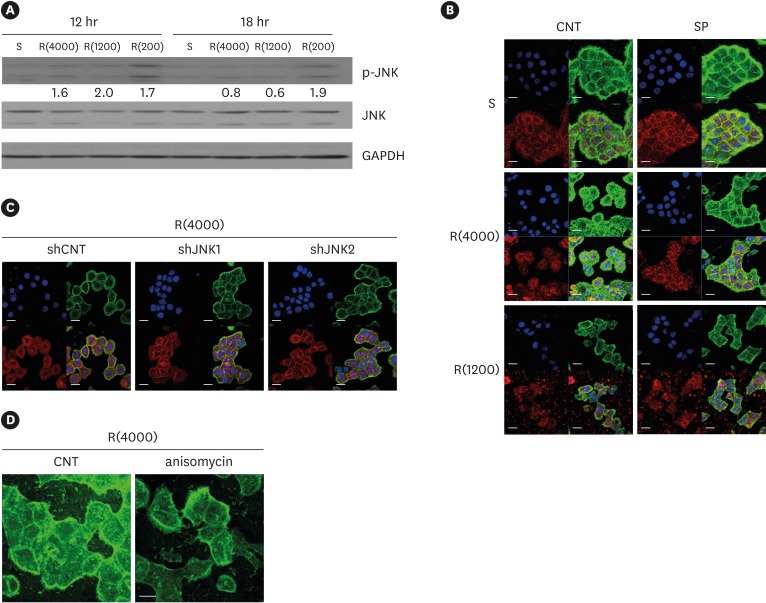
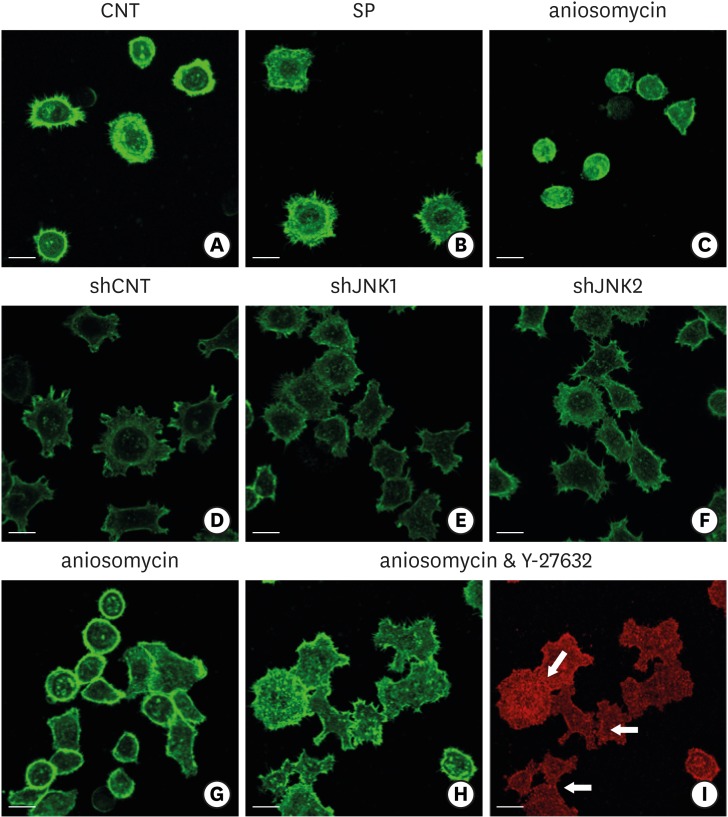
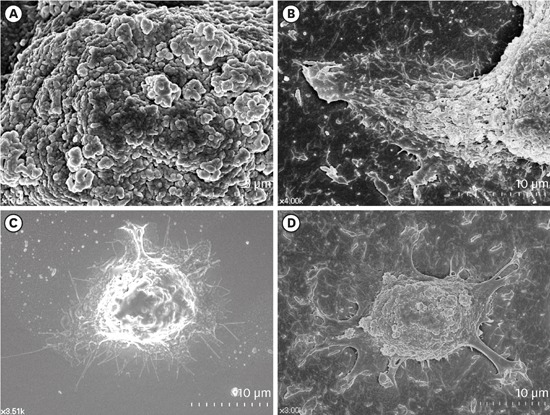
9. Lee G, Kim HJ, Kim HM. RhoA-JNK regulates the E-cadherin junctions of human gingival epithelial cells. J Dent Res. 2016; 95:284–291. PMID:
26635280.

10. Zheng L, Kim HM. Low-Rac1 activity downregulates MC3T3-E1 osteoblastic cell motility on a nanoscale topography prepared on polystyrene substrates
in vitro
. J Biomed Mater Res A. 2013; 101:1629–1636. PMID:
23184573.
11. Torres-Gallegos I, Zavala-Alonso V, Patiño-Marín N, Martinez-Castañon GA, Anusavice K, Loyola-Rodríguez JP. Enamel roughness and depth profile after phosphoric acid etching of healthy and fluorotic enamel. Aust Dent J. 2012; 57:151–156. PMID:
22624754.

12. Barkmeier WW, Erickson RL, Kimmes NS, Latta MA, Wilwerding TM. Effect of enamel etching time on roughness and bond strength. Oper Dent. 2009; 34:217–222. PMID:
19363978.

13. Singh S, Uppoor A, Nayak D. A comparative evaluation of the efficacy of manual, magnetostrictive and piezoelectric ultrasonic instruments--an
in vitro profilometric and SEM study. J Appl Oral Sci. 2012; 20:21–26. PMID:
22437673.
14. Vastardis S, Yukna RA, Rice DA, Mercante D. Root surface removal and resultant surface texture with diamond-coated ultrasonic inserts: an
in vitro and SEM study. J Clin Periodontol. 2005; 32:467–473. PMID:
15842261.
15. Park NH, Min BM, Li SL, Huang MZ, Cherick HM, Doniger J. Immortalization of normal human oral keratinocytes with type 16 human papillomavirus. Carcinogenesis. 1991; 12:1627–1631. PMID:
1654226.

16. Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol Biol Cell. 2007; 18:3429–3439. PMID:
17596509.

17. Lecuit T. “Developmental mechanics”: cellular patterns controlled by adhesion, cortical tension and cell division. HFSP J. 2008; 2:72–78. PMID:
19404474.

18. Maghzal N, Kayali HA, Rohani N, Kajava AV, Fagotto F. EpCAM controls actomyosin contractility and cell adhesion by direct inhibition of PKC. Dev Cell. 2013; 27:263–277. PMID:
24183651.

19. Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002; 4:408–415. PMID:
11992112.

20. Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003; 424:219–223. PMID:
12853963.

21. Otto IM, Raabe T, Rennefahrt UE, Bork P, Rapp UR, Kerkhoff E. The p150-Spir protein provides a link between c-Jun N-terminal kinase function and actin reorganization. Curr Biol. 2000; 10:345–348. PMID:
10744979.

22. Samak G, Gangwar R, Crosby LM, Desai LP, Wilhelm K, Waters CM, et al. Cyclic stretch disrupts apical junctional complexes in Caco-2 cell monolayers by a JNK-2-, c-Src-, and MLCK-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2014; 306:G947–G958. PMID:
24722904.
23. Hamel M, Kanyi D, Cipolle MD, Lowe-Krentz L. Active stress kinases in proliferating endothelial cells associated with cytoskeletal structures. Endothelium. 2006; 13:157–170. PMID:
16840172.

24. Mengistu M, Brotzman H, Ghadiali S, Lowe-Krentz L. Fluid shear stress-induced JNK activity leads to actin remodeling for cell alignment. J Cell Physiol. 2011; 226:110–121. PMID:
20626006.

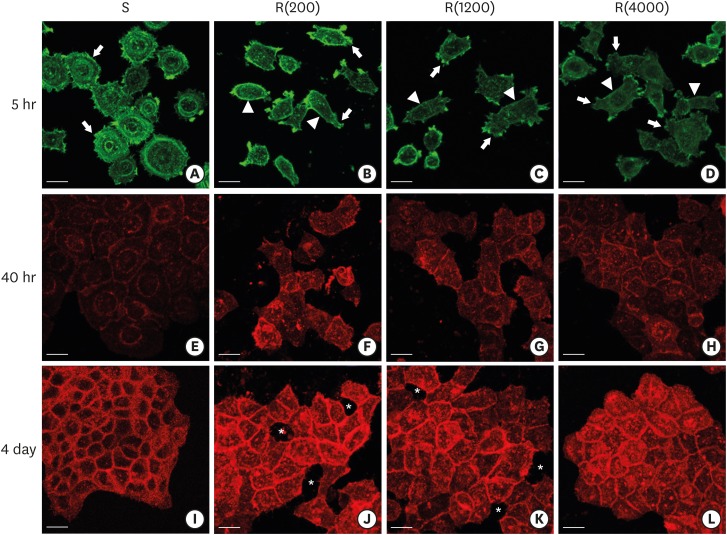
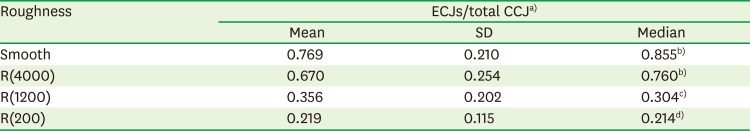
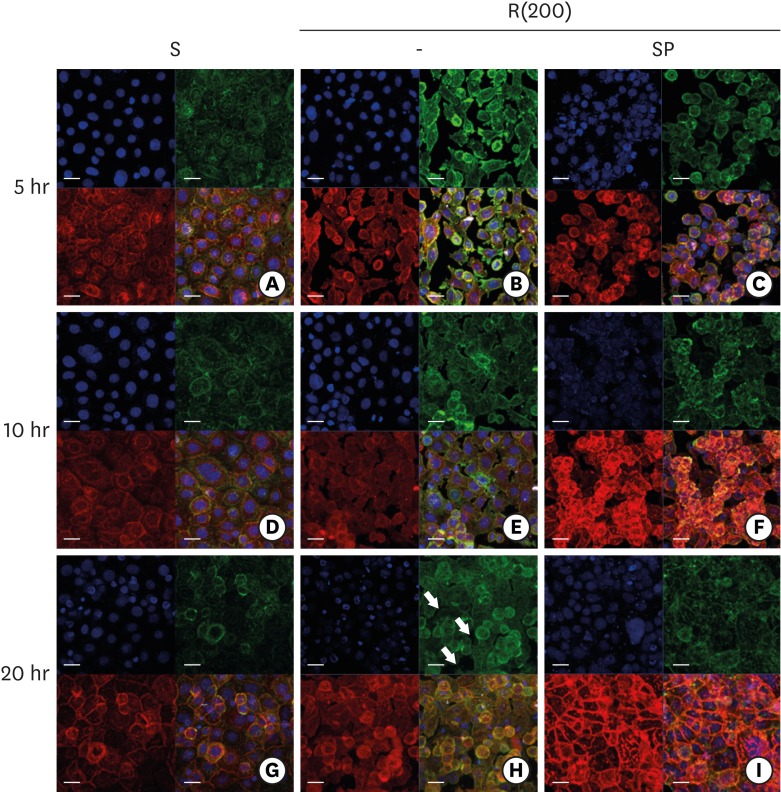
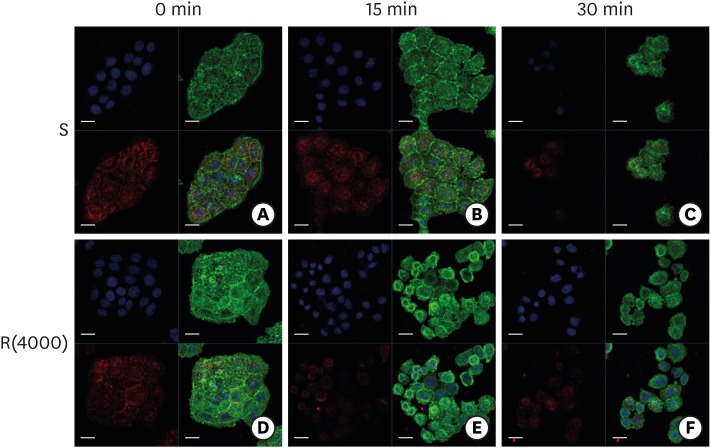




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download