INTRODUCTION
In 1957, Barber used the term polymyalgia rheumatica (PMR) to describe sudden onset of inflammatory features characterized by shoulder and pelvic girdle pain and stiffness.
1 Since that description, PMR has become a common systemic disease in the elderly, characterized by inflammatory pain and stiffness of the shoulder and/or pelvic girdles combined with laboratory evidence of inflammation.
234
There is lack of standardized diagnostic criteria for PMR, but sets of classification criteria have been proposed by several groups of investigators. Recently, the 2012 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria were developed to standardize the definition of PMR and thereby address the uncertainty that commonly surrounds its diagnosis.
5 Although the 2012 EULAR/ACR classification criteria were developed, chronic inflammatory and autoimmune disease, degenerative disease, infections, and malignancies can mimic PMR. Heterogeneity in clinical manifestation and the lack of diagnostic criteria of PMR have led to misdiagnosis.
There are many reports on the various symptoms of PMR. The clinical features that appear in PMR vary by country.
467 Bilateral shoulder pain with morning stiffness is the reported symptom in 70%–95% of PMR patients.
2 Peripheral arthritis was reported in 25% of patients, while 40% of PMR patients have constitutional manifestations including low-grade fever, depression, fatigue, and weight loss.
389 In previous Korean epidemiology studies, the incidence of constitutional symptoms and peripheral arthritis was similar to those reported in Caucasian populations. However, the incidence of hip girdle pain was higher in the Korean studies than reported from western countries.
610 In addition, western population studies reported concomitant giant cell arteritis (GCA) in 16%–21% of the study population.
11 However, no GCA case were reported in the Korean epidemiology study populations.
610
Although PMR is well controlled by a low dose of oral glucocorticoid, not all patients respond adequately, and relapse and long-term glucocorticoid dependency are common.
12 The treatment response to oral prednisolone for PMR also varies. In a prospective western population cohort study of PMR, 56% of prednisolone-treated patients achieved complete response.
13 However, in previous Korean studies, the remission rate was reported as about 20%.
610 Demographic and clinical characteristics, along with outcomes, in PMR patients have been studied extensively in western countries. However, Korean PMR patient studies are still sparse.
The aim of our study was to investigate the clinical and demographic characteristics, including related factors, of therapeutic responses in Korean PMR patients.
DISCUSSION
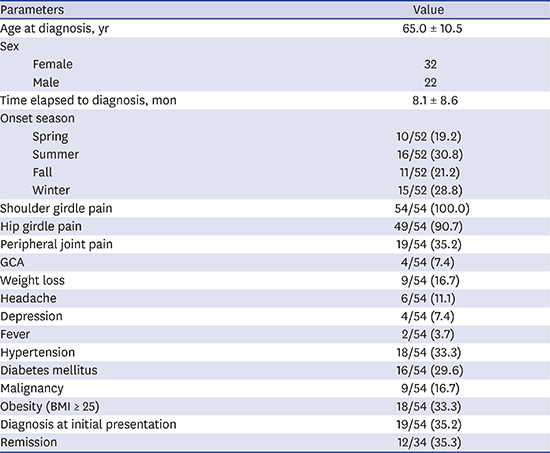
In this study, we described the demographic and clinical features of 54 Korean patients with PMR. Furthermore, we analyzed the therapeutic response to oral prednisolone and predictive factors of remission-failure. The rate of remission on oral prednisolone treatment was 35.3%, and history of relapse was a predictor of remission-failure. Four of 54 patients (7.4%) were also diagnosed with GCA.
In our study, 12 of 34 patients (35.3%) were in remission after oral prednisolone treatment at the one-year follow-up. Twenty-three of 34 patients (67.6%) had relapsed at least once during treatment. Because relapse and long-term glucocorticoid dependency are common, remission is difficult to achieve in PMR treatment.
1417 The remission rate of our study was 35.3%, which is comparable to previous reports in western countries. In 94 Italian PMR subjects, Salvarani et al.
18 had found that 50% of patients were in remission after oral prednisolone at a mean starting dosage of 17.5 mg/day. In addition, a multicenter prospective cohort study in 10 European countries and the United States reported a 56% complete response rate to corticosteroids at week 26. In contrast, previous Korean studies have reported remission rates of 19.5%–20.5%, which is lower than the rate in our study. In 10 multicenter study of 51 Korean subjects, the remission rate was 19.5%.
10 Lee et al.
6 evaluated the clinical data of 78 patients with PMR from 5 hospitals and reported a 20.5% remission rate. One explanation for these lower rates is that in previous multicenter-based Korean studies, the treatment strategies of PMR varied between centers, and the initial oral prednisolone dosage and the tapering schedule were not standardized. Another possible explanation is that the definition of remission among studies was not consistent. In our study, we standardized assessment and the initial dose and tapering schedule of oral prednisolone according to the BSR and the BHPR guidelines. This standardization may have led to better outcome in comparison to previous Korean studies. Our findings indicate that the remission rate of PMR patients on oral prednisolone is not low in the Korean population.
GCA and PMR are conditions of elderly that frequently overlap. GCA predominantly affects large- and medium-sized blood vessels with systemic vasculitis in individuals over the age of 50. The most commonly affected areas are the aorta and its major braches.
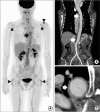
19 It is associated with increased thrombotic events, both arterial and venous thromboses. Aortic involvement usually remains unnoticed until there are fatal complications. The diagnosis of GCA is based on clinical symptoms and laboratory findings consistent with systemic inflammation and confirmed with histologic analysis. Also, FDG-PET/CT has a major role in assessing the extent of disease.
20 Previous western population studies have shown that GCA is present in 16%–21% of PMR patients.
11 However, in epidemiology studies on PMR patients within the Korean population, no GCA cases were reported.
610 In our present study, four patients (7.4%) were diagnosed with GCA. One patient was clinically diagnosed and three patients were diagnosed with characteristic findings of GCA, increased FDG uptake in the aorta and its major branch vessel as determined by FDG-PET/CT. In this regard, GCA is not a rare condition in Korean PMR patients. Therefore, all patients with PMR should be carefully assessed for GCA.
In this study, history of relapse was a significant independent risk factor (
P < 0.05) for remission-failure of PMR (OR, 6.81; 95% CI, 1.035–44.804). In previous studies, high initial acute phase reactant level, female sex, older age, and longer duration of symptoms were reported as prognostic factors for treatment response.
21 However, the factors related to treatment are still not clear. Our study showed that older age, female sex, diabetes mellitus, malignancy, and initially high levels of CRP and ESR were not risk factors of remission failure in univariate logistic analysis. History of relapse was the only significant predictor of remission-failure in multivariate analysis (OR, 6.81; 95% CI, 1.035–44.804). In our study, 23 of 34 patients (67.6%) had relapsed at least once during oral prednisolone treatment. This is consistent with a previous report; Kim et al.
10 found that the relapse rate was 68.8% and the frequency of relapse was significantly higher in patients without remission (
P < 0.02). These findings suggest that the presence of relapse might be an important parameter for predicting remission in PMR patients. Larger prospective studies are needed to determine predictive factors of remission in PMR patients.
The treatment for PMR is primarily based on oral glucocorticoid. The EULAR and the ACR recommend oral glucocorticoid treatment in PMR for a minimum of 12 months.
12 Long-term treatment of glucocorticoid treatment is related to cardiovascular side effects and risk factors for the development of glucocorticoid-induced diabetes mellitus. Patients with an older age, higher HbA1c level and lower eGFR require close monitoring for the development of glucocorticoid-induced diabetes mellitus, regardless of the dose of glucocorticoids.
22 Also, glucocorticoid induced osteoporosis is the most common cause of secondary osteoporosis. A treatment with 10 mg/day of prednisone or equivalent for more than 3 months leads to a 7-fold increase in hip fractures and a 17-fold increase in vertebral fractures.
23 In a large cohort of patients from the National Database of the German Collaborative Arthritis Centers, median glucocorticoid doses ≤ 5 mg/day were reached at a 13–18 months disease duration in PMR patients. And the prevalence of osteoporosis increased within 3 years in a national rheumatology database.
24 To prevent glucocorticoid induced osteoporosis the use of bone protection when initiating glucocorticoid treatment for PMR is recommended.
14 We used of vitamin D and bisphosphonates routinely when initiating oral prednisolone for PMR to prevent the complications of osteoporosis and monitoring for the development of glucocorticoid-induced diabetes mellitus by blood sugar test and HbA1c level. PMR requires long-term glucocorticoid treatment longer than. If 1-year and patients are older, careful examination and monitoring of the side effects of glucocorticoid is necessary.
The diagnosis of PMR is clinically based on proximal inflammatory pain associated with increased ESR and/or CRP in the elderly. Elderly onset RA, late onset spondyloarthropathy, inflammatory myopathies, adhesive capsulitis, fibromyalgia, subacute infections, and occult malignancies can mimic PMR.
25 In PMR patients, magnetic resonance imaging or ultrasonography typically shows bilateral bursitis or synovitis in the hips or shoulders.
23 However, these radiologic findings may also occur in other inflammatory or degenerative joint diseases. In our study, only 19 patients (35.2%) were diagnosed with PMR when symptoms initially presented. Twenty-six patients (48.1%) had varying previous diagnoses before visiting our hospital. Nine patients (16.7%) had no specific diagnosis prior to diagnoses of PMR at our facility. Because of inflammatory nature of the spinal pain, 6 patients (11.1%) were misdiagnosed as spondyloarthropathy. Clinical features such as limitation of motion in the shoulder and pain aggravated by movement may lead to a misdiagnosis of adhesive capsulitis; this was the case for 6 patients (11.1%) in this study. Elevated acute phase reactants in PMR can mimic an infectious disease such as infectious arthritis or lumbar osteomyelitis. Three patients fell into this category in our study (5.6%). There are several explanations for misdiagnosis of PMR. As previously mentioned, it is difficult to exclude a variety of conditions that mimic the clinical features of PMR. New biomarkers and imaging techniques specifically related to PMR would be useful to clarify these clinical features. Lower incidence of PMR may be attributable to a lack of awareness on the part of physicians. A precise and specific approach is essential for the accurate diagnosis of PMR, and higher physician awareness may be needed.
In this present study, morning stiffness was reported in 48 patients, and it lasted 45 minutes or more in 38 patients (76%). Morning stiffness is one of the classification criteria for PMR, but the durations vary depending on the classification criterion and include > 1 hour, > 30 minutes, presence, not included as criterion.
26 The 2012 EULAR/ACR criteria include > 45 minutes of morning stiffness as 2 of the clinical criteria points, and there is no consideration given to a shorter duration of morning stiffness. In this study, 20.0% of patients suffered from morning stiffness for less than 45 minutes, and 4.0% of patients did not complain of morning stiffness. Morning stiffness should be included as one classification criterion; however, 45 minutes may be too long and might decrease the sensitivity of PMR diagnosis.
Interestingly, the incidence of hip girdle pain (90.7%) in our study was higher than the incidence in previous studies.
78102728 Mori et al.
8 reported that 34% of Japanese PMR patients showed pelvic girdle pain, and a study of Korean PMR patients reported 59.6% pelvic girdle pain.
10 In this study, positive provocation tests for the hip girdle, such as the Patrick test and evoked pain during squatting, were included in hip girdle pain. Provocation tests for the hip girdle could explain the higher incidence of hip girdle pain in our study. Hip girdle pain is one of the clinical features of PMR, and the 2012 EULAR/ACR classification criteria highlight the importance of hip involvement since it is 1 point on the clinical criteria. Thus, the provocation test for hip pain could be useful to facilitate diagnosis of PMR.
The incidence of PMR appears to vary in the world, with higher rates observed in studies performed in northern European populations. Variation in incidence suggests environmental factors, such as seasonal effects. Indeed, previous epidemiological studies have shown a regular cyclical pattern of PMR incidence. In a retrospective study of 201 PMR patients, the onset of PMR showed a significant winter periodicity.
29 Also, Kim et al.
10 reported that 45.1% of PMR patients were diagnosed in winter. A possible mechanism suggests a role for precipitating environmental factors, such as
Mycoplasma pneumoniae,
Chlamydia pneumoniae, and parvovirus B19. Moreover, some authors have reported seasonal variation in the immune system and inflammatory responsiveness associated with several infectious agents. However, in the present study, no seasonal preferences were observed. In several studies, authors had failed to observe seasonal variation and infectious etiology associated with PMR onset.
3031 Cimmino et al.
32 had found 62% of PMR cases developed in the summer, suggesting that onset of PMR correlated with outside temperature and hours of sunshine. Peris
31 had reported that onset of PMR symptoms is unrelated to the season, and Parvovirus B19 infection was unrelated to PMR onset in a 4-year prospective study. Our study findings do not support a seasonal pattern for PMR diagnosis. Since studies on seasonal patterns and PMR onset have been inconsistent, a large prospective study is needed to estimate seasonal variation.
The BSR and the BHPR recommended that atypical presentations, such as patients younger than 60 years and those with peripheral inflammatory arthritis, systemic symptoms, or very high or normal inflammatory markers, should be considered for early specialist referral, including those patients with incomplete glucocorticoid response or at high risk of therapy-related side effects.
1214 In our study, six PMR patients (11.1%) were referred to a rheumatologic specialist for further treatment. Three of six patients were also diagnosed with GCA. Remaining three patients were refractory to prednisolone therapy with uncontrolled musculoskeletal pain.
There were several limitations of this study. The first is the retrospective study design. Although we reviewed the past 8 years of data, we had some insufficient demographic data, clinical characteristics and comorbidities such as cardiovascular disease and osteoporosis. A second limitation is that 20 patients were excluded in the analysis of response to oral prednisolone treatment. And, we collected data of PMR for more than 8 years, but did not have large sample sized because our retrospective study is a single center experience. Due to small sample size, in logistic regression to analysis of predictive factors of treatment response the range of 95% CI is wide. Despite these limitations, the present study identifies more precise clinical characteristics and therapeutic response in PMR, because of the relatively large sample size in an Asian population and a standardized treatment protocol for PMR. Larger prospective studies will be needed to precisely ascertain the clinical characteristics and therapeutic response in PMR.
In conclusion, the rate of remission (35.3%) after oral prednisolone treatment was similar to previous reports from western countries, and GCA is not a rare condition in Korean PMR patients. Misdiagnosis of PMR is common; therefore, heightened awareness of PMR is needed in elderly patients with bilateral hip and/or shoulder pain. Physicians dealing with locomotive system pain should always consider PMR in elderly patients who present with the inflammatory feature of bilateral shoulder pain.










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download