INTRODUCTION
Schistosomiasis is the most prevalent trematodiasis globally, caused by blood flukes of the genus
Schistosoma. In particular,
Schistosoma haematobium causes urogenital schistosomiasis (UGS) widely in Africa by worms residing in the venous plexus of the urinary bladder and its eggs deposited in the bladder wall.
1 Sudan is an endemic area of UGS where the White Nile and Blue Nile rivers meet. In Sudan, there are several known hyperendemic locations of UGS.
234 Previous studies have shown that the prevalence of
S. haematobium in White Nile State, Sudan remained high, with 45.5% in 2010,
4 28.5% in 2012,
5 and 14.0% in 2014.
6
As schistosomiasis is a neglected tropical disease (NTD) of global importance, it is one of target NTDs for global elimination programs. The elimination programs of NTDs must be supported by mass diagnostic screening and preventive chemotherapy. Therefore, it is important to implement accessible and inexpensive mass diagnostic screening test with high sensitivity. Urine microscopy (UM) to detect and count the eggs of
S. haematobium has been used widely as a standard diagnostic test of UGS because it is easy and cheap to perform in remote endemic areas. However, the eggs are not regularly passed through the urine because the eggs are released only when the infected blood vessels rupture into the bladder lumen. Therefore, UM used to underestimate UGS prevalence especially in detecting light infections.
7 In this context, additional methods are applied together to improve the diagnosis of UGS such as ultrasonography,
6 dipstick for hematuria,
89 and serology.
8910
In the present study, we applied enzyme-linked immunosorbent assay (ELISA) for diagnosis of UGS by detecting the serum immunoglobulin (Ig) G antibodies reacting to soluble egg antigen of S. haematobium as well as UM. Our study aimed to establish the criteria of ELISA diagnosis and to compare diagnostic values of UM and ELISA for UGS.
DISCUSSION
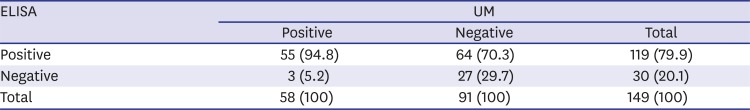
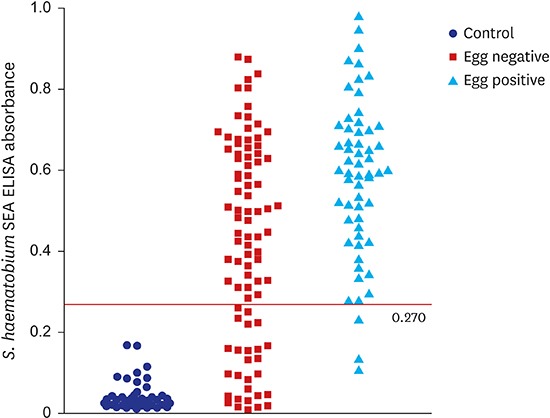
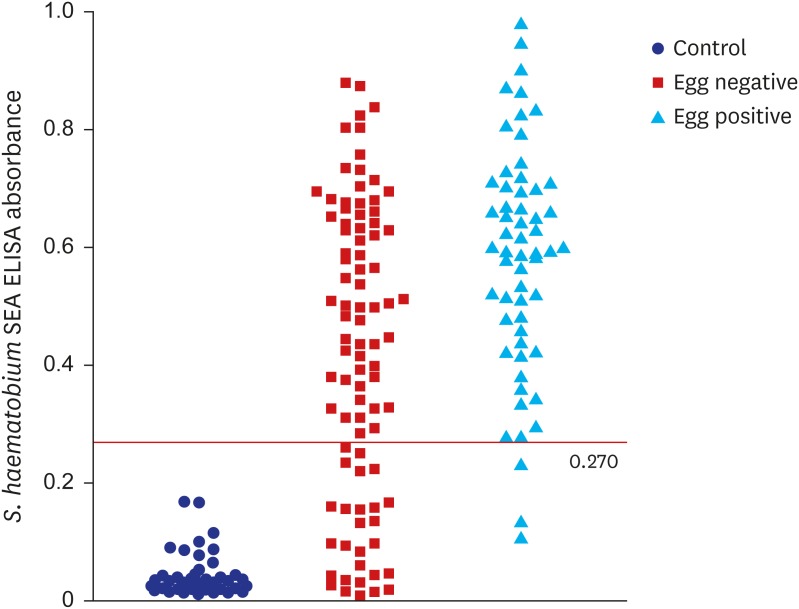
The present sensitivity of ELISA was high enough to apply this method practically to screen egg positive subjects. In this context, the optimum cut-off value should include as many egg-positive samples while excluding as many egg-negative samples. To achieve this, cut-off value is usually adjusted to maximize Youden index, but this strategy has limitations by giving equal weight on sensitivity and specificity, which is not optimal in a condition of relatively low prevalence and higher cost of false-negative test results than that of false-positive test results.
13 Therefore, the cut-off value was selected among values with sensitivity higher than 90.0%. As a result, the absorbance of 0.270 was set as the cut-off value for positive criteria (
Fig. 3), which meant samples with ELISA absorbance higher than 0.270 were counted positive by our in-house serology system. Although distribution of the ELISA absorbance values was significantly different between the UM negative and positive groups (
P < 0.001), the cut-off value of 0.270 should be reset with more number of positive and negative samples due to high rate of irrelevance between the two methods (
Fig. 3 and
Table 2).
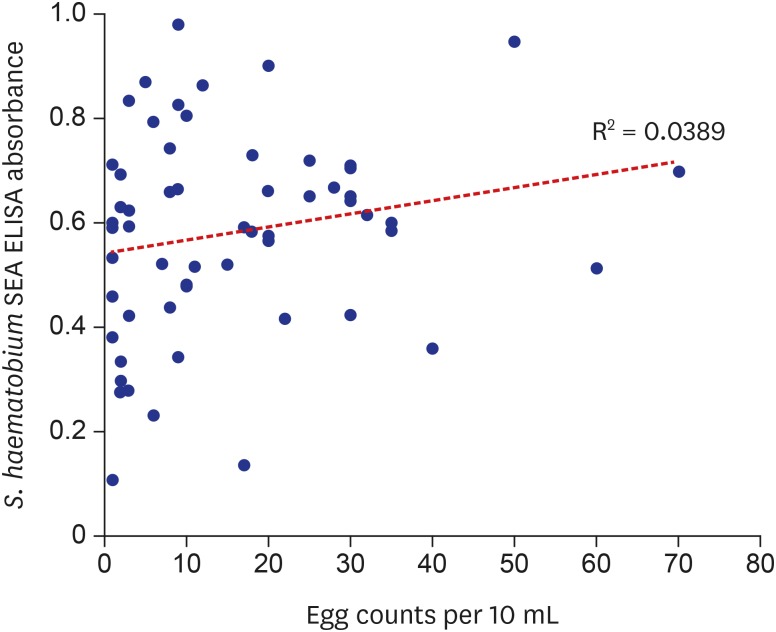
In spite of high sensitivity, three were false ELISA negatives among 58 egg positives. One of the three had 0.231 of S. haematobium ELISA absorbance and adjusting the cut-off value to 0.230 could increase sensitivity to 96.6% but it also impaired the specificity to 26.4%. The rest two had 0.135 and 0.107 of S. haematobium ELISA absorbance but had 0.244 and 0.233 absorbance to S. mansoni antigen respectively. The absorbance to S. mansoni antigen was higher than that to S. haematobium, but significance of the difference is unclear. Immune status or other factors must be further investigated in these false negative subjects by ELISA.
The sensitivity and specificity of ELISA can be influenced by types of antigen utilized. One study recognized that ELISA using worm antigen of
S. mansoni could screen UGS by detecting serum IgG antibodies but its sensitivity was not high.
8 Instead, the ELISA detecting serum IgM antibodies against egg antigens showed high sensitivity but low specificity.
910 It is hard to directly compare the serology findings between IgG or IgM antibodies, but our ELISA detecting serum IgG antibodies against egg antigens demonstrated similarly high sensitivity and low specificity. Since host immune response mainly targets the egg antigen rather than the worm-derived antigen, it is reasonable to employ soluble egg antigen for ELISA evaluation.
The specificity of ELISA was only 29.7%, and 64 subjects (70.3%) in UM negative group were found to be positive by ELISA. These subjects may be false positive cases of cured UGS, true infected with negative eggs by UM at the collection time, or false positive by cross-reaction.
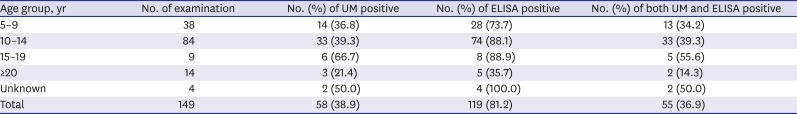
14 Chemotherapy with praziquantel may kill adult flukes but the eggs deposited in the bladder wall or in other tissues can sustain longer. Because several mass drug administration (MDA) programs have been implemented in Sudan, the cured subjects are diagnosed as false positive by ELISA due to sustaining eggs in the tissue and long-lasting residual antibodies in the serum. When the subjects were divided by age group, the proportion of ELISA positive in UM negative group reached 100% and 80.4% in the age group of 15–19 year and 10–14 year, respectively. Because these age groups are major targets of several school-based MDA programs, most-reaction by
S. mansoni infection or other multi-parasitism. All of the 3 possible factors might have contributed the low specificity of ELISA in the present study.
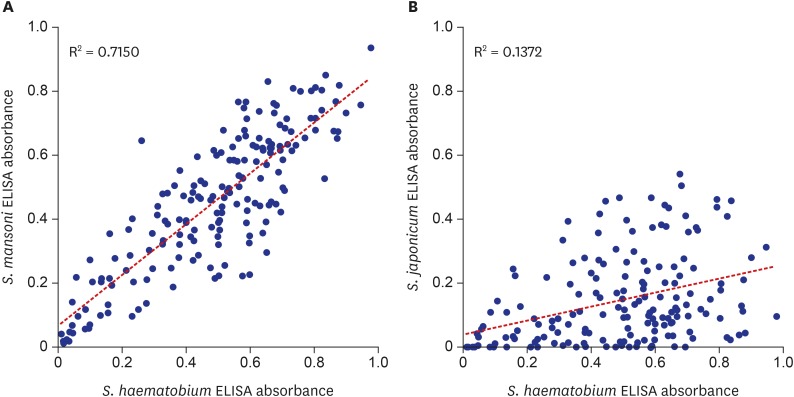
One important factor to consider for ELISA diagnosis is cross-reaction. Serum IgG antibodies to other helminth antigens may falsely react to the egg antigen of
S. haematobium and the antibodies to
S. haematobium cross-react with antigens of other helminths. Indeed, significant correlation between ELISA results using antigens from different schistosome species (
Fig. 5) infers the
S. haematobium infected serum frequently cross-reacted with the soluble egg antigens of
S. mansoni and
S. japonicum. The cross-reaction between
S. haematobium and
S. mansoni was also recognized in other studies.
810 On the other hand, there is a chance of true multiple infections because mixed infections with
S. mansoni and
S. haematobium were reported in the study site of Sudan.
45 However, most positive reactions against
S. mansoni antigen must have been contributed by cross-reaction because
S. mansoni prevalence is very low. Similarly, the ELISA positivity to
S. japonicum mostly resulted from cross-reaction because the species is distributed only in Asia. This extraordinary frequency of cross-reaction within the
Schistosoma species must be concerned for developing and interpreting ELISA diagnosis of schistosomiais.
In addition, we should consider temporal changes of ELISA results in response to the infection and treatment. Since the ELISA employed soluble egg antigen, certain amounts of accumulated eggs in the bladder wall or other tissues are required for the ELISA positive reaction. During the initial infection, the infected subjects can show negative results by ELISA until enough numbers of eggs accumulate in the bladder wall. During the treatment, the eggs in the tissue can last for a long period of several years even after cure by medication. A multiplex bead assay for serodiagnosis of
S. mansoni infection in Kenya reported high positivity to soluble egg antigen among children and unchanged positivity for 3 years after medication.
15 During a certain period after medication, the ELISA diagnosis can lead to false positive results according to the disease nature of UGS.
Soluble egg antigens can be prepared from different isolates of S. haematobium. The present study used soluble egg antigen derived from Egyptian isolate of S. haematobium. It is unknown whether there is any inter-isolate difference of egg antigens by locality between Sudan and Egypt or other areas. The antigenic characteristics of soluble egg antigen of S. haematobium must be specified in the near future according to geographical distribution and species of Schistosoma.
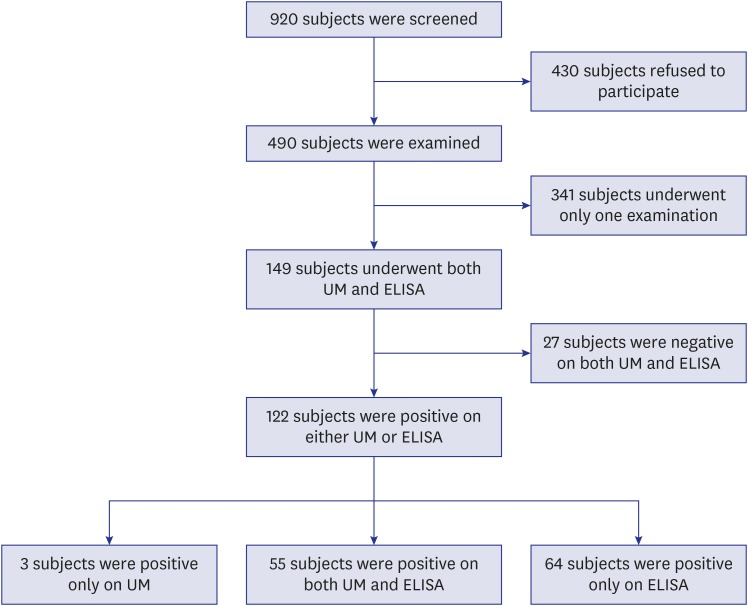
The present study has several limitations. The sample size was small due to many lost subjects for the tests and the subjects were not sampled by statistical design. It was impossible to prepare the gold standard by UM because the samples were collected once. For further evaluation of diagnostic efficacy of UM and ELISA, collection of whole day urine or more repeated collections on successive days should be considered.
In conclusion, we have established in-house ELISA with cut-off absorbance 0.270 for screening serum IgG antibodies by employing soluble egg antigen of S. haematobium for diagnosis of UGS. Diagnostic sensitivity of the ELISA was 94.8% and specificity was 29.7%. The ELISA diagnosis can supplement the conventional diagnosis of UGS by UM.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download