Abstract
Ventricular septal rupture (VSR) is a disastrous mechanical complication of myocardial infarction. Although several surgical interventions have been developed, mortality due to surgical management remains high, especially in the case of posterior VSR. We report a successful case of repair of posterior VSR using an alternative transatrial approach to avoid the complications related to ventricular incision.
Although several surgical interventions have been developed, mortality and complications related to surgical management remains high, especially with respect to trans-ventricular approach. Ventricular incision also has several disadvantages: increased postoperative bleeding, ventricular malfunction, and ventricular arrhythmia. In the present work, we report a successful case of repair of posterior ventricular septal rupture (VSR) via transatrial approach.
A 73-year-old woman presented to a local hospital with shortness of breath and anterior chest pain. The electrocardiogram (ECG) demonstrated ST-segment elevations in the inferior leads, but regional wall motion abnormality was not noted on the transthoracic echocardiogram. The coronary angiogram showed severe stenosis at the middle of the right coronary artery (Fig. 1). The left coronary catheterization revealed mild stenosis in the middle left anterior descending coronary artery. Percutaneous coronary intervention (PCI) was performed at the right coronary artery by stent insertion. After PCI, sinus tachycardia changed to ectopic junctional rhythm on the ECG, and severe hypotension was noted. The additional transthoracic echocardiogram revealed VSR at the basal inferoseptal wall and severe tricuspid regurgitation with right ventricular dilation (Fig. 2). Consequently, the patient was transferred to our hospital for operative management. Intraaortic balloon pump (IABP) insertion was followed by emergent operation. A median sternotomy was performed, and cardiopulmonary bypass (CPB) was established via ascending aortic and bicaval cannulation. Simultaneously, the saphenous vein was harvested at the left calf. The right atrium was opened along the side of the atrioventricular groove. VSR was noted at the basal inferior septum measuring approximately 1.5 cm in diameter (Fig. 3). The septal and posterior leaflet and a few chordae were resected for more consistent access to the VSR. Necrotic tissue was debrided from the interventricular septum. Eleven stitches of pledget buttressed by 2-0 prolen were sutured by the method of interrupted horizontal mattress along the ventricular septal defect (VSD) margin. The stitches near the septal leaflet were sutured through the annulus of the septal leaflet, and the stitches for the inferior margin were sutured through the inferior free wall of the right ventricle. These stitches were passed through the trimmed Teflon felt and tied into place without difficulty. After VSR closure, the anterior and posterior leaflets were reattached to the annulus. Tricuspid valve replacement was performed with a Carpentier Edward valve (Edwards Lifesciences, Irvine, CA, USA) by using a supra-annular technique (Fig. 4). Although PCI for right coronary artery lesion was done successfully, we decided to perform a right coronary artery bypass to be prepared for the possibility of acute thrombosis. The distal right coronary artery was bypassed with a saphenous venous graft in an end-to-side fashion. The patient was weaned off CPB without complications. The immediate postoperative transthoracic echocardiogram (TTE) revealed right ventricular dysfunction, and the IABP was removed from the patient on postoperative day 4. The patient was discharged 29 days after the operation. There was no evidence of residual VSR, and right ventricular function was in the normal range on the TTE.
Post-infarct VSD occurs in only 1-2% of myocardial infarction patients. Several risk factors are associated with post-infarct VSR: first infarction, inferior location, transmural infarction, complete and sudden occlusion of a coronary artery, poor collateral blood flow, and left ventricular hypertrophy.1)2) With the development of percutaneous coronary intervention, the incidence of postinfarct VSR has been reduced; however, mortality remains high.3) Post-infarct VSR is associated with an 87% mortality within 2 months if managed medically.4) Previously, many surgeons delay surgical intervention while waiting for myocardial fibrosis to occur because this facilitates surgical repair. More recently, the majority of surgeons have advocated more aggressive management, with immediate start of IABP and urgent repair of VSR. In general, most surgeons conduct the operation via left ventriculotomy or via the infarcted free wall. However, in the case of posterior VSR, access is difficult through left ventriculotomy or infarcted free wall.5) Ventricular incision also has several disadvantages: increased postoperative bleeding, ventricular malfunction, and ventricular arrhythmia. In 1986, Filgueira et al.6) described the transatrial approach to repair the VSR in order to avoid some of the problems of ventricular incision. However, the transatrial approach is also associated with certain problems. It is difficult to expose the VSR and to accurately identify the VSD due to the trabeculation of the right ventricle. In addition, the placement of the stitch through the tricuspid valve may interfere with the subvalvular apparatus, and tricuspid regurgitation can occur. These limitations can be overcome by several methods. Wide right atriotomy and multiple stay sutures can provide appropriate exposure. Additional exposure can be achieved by detaching the tricuspid leaflet from the annulus. However, the tricuspid valvectomy method requires tricuspid valve repair after VSR repair.
In the present case, we performed a tricuspid valve replacement instead of a repair to avoid the risk of tricuspid regurgitation that might occur if the valvular mechanism is compromised during exposure and patch closure of the VSR. Moreover, inferior myocardial infarctions may result in right ventricular dysfunction and low cardiac output syndrome postoperatively, which can worsen due to residual tricuspid regurgitation. Accurate identification of VSR is crucial to avoid residual shunt flow and can be achieved by injecting blood-tinged saline solution through a left ventricular vent catheter placed via the right superior pulmonary vein. Few studies have reported on the transatrial approach. Massetti et al.7) found that posterior VSR could be successfully repaired via the transatrial approach. By avoiding additional damage to the ventricle, it reduces the risks of postoperative bleeding and enhances survival. Lee et al.8) reported a successful right atrial approach operation for post-infarction rupture of the posterior ventricular septum. However, unlike in our case, the operation was conducted 12 days after infarction.
In conclusion, many surgeons advocate the use of ventriculostomy or infarcted tissue for the treatment of postinfarct VSR; however, posterior septal rupture can be repaired successfully through an alternative transatrial approach, thus avoiding the complications that can occur due to ventriculotomy.
Figures and Tables
Fig. 1
Coronary angiogram. Left anterior oblique cranial view shows near total occlusion at the middle right coronary artery.
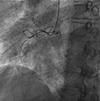
Fig. 2
Transthoracic echocardiogram (TTE). A preoperative TTE reveals shunt flow through the ventricular septal rupture. RV: right ventricle, LV: left ventricle.
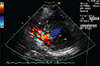
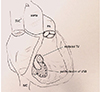
Fig. 3
Operative findings. Identification of the posterior postinfarction VSD through right atrium and tricuspid valvectomy. CS: coronary sinus, IVC: inferior vena cava, SVC: superior vena cava, PL: posterior leaflet, RAA: right atrial appendage, RV: right ventricle, SL: septal leaflet, VSD: ventricular septal defect.

References
1. Raddford MJ, Johnson RA, Daggett WM Jr, et al. Ventricular septal rupture: a review of clinical and physiological features and an analysis of survival. Circulation. 1981; 64:545–553.
2. Muehrcke DD, Daggett WM. Current surgical approach to acute ventricular septal rupture. Adv Card Surg. 1995; 6:69–90.
3. Coskun KO, Coskun ST, Popov AF, et al. Experiences with surgical treatment of ventricle septal defect as a post infarction complication. J Cardiothorac Surg. 2009; 4:3.
4. Komeda M, Fremes SE, David TE. Surgical repair of postinfarction ventricular septal defect. Circulation. 1990; 82:5 Suppl. IV243–IV247.
5. Kirklin JW, Barratt-Boyes BG. Postinfarction ventricular septal defect. Cardiac surgery. New York: Churchill Livingstone;1993. p. 403–413.
6. Filgueira JL, Battistessa SA, Estable H, Lorenzo A, Cassinelli M, Scola R. Delayed repair of an acquired posteior septal defect through a right atrial apporoach. Ann Thorac Surg. 1986; 42:208–209.
7. Massetti M, Babatasi G, Le Page O, Bhoyroo S, Saloux E, Khayat A. Postinfarction ventricular septal rupture: early repair through the right atrial approach. J Thorac Cardiovasc Surg. 2000; 119(4 Pt 1):784–789.
8. Lee WY, Kim SJ, Kim KI, et al. Transatrial repair of post-infarction posterior ventricular septal rupture. Korean J Thorac Cardiovasc Surg. 2011; 44:186–188.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download