Abstract
Background
The SD Bioline Strep A Ultra (SD, Yongin, Korea) is a recently developed rapid antigen detection test (RADT) for diagnosing bacterial pharyngitis caused by Group A Streptococcus, We evaluated the performance of SD Bioline Strep A Ultra, using the number of colony forming units and color intensity.
Methods
Three throat swabs each were taken from 343 children with pharyngitis who visited pediatric clinics. We evaluated the performance of SD Bioline Strep A Ultra and compared its positive rate with the number of colony forming units, using the Fisher exact test.
Results
The sensitivity, specificity, positive predictive value, and negative predictive value (95% confidence interval) were 97.4% (94.0–99.1%), 90.8% (85.0–94.9%), 93.0% (88.5–96.1%), and 96.5% (92.0–98.9%), respectively. Positive rate significantly differed by number of colony forming units (P=0.021). ROC plot for color intensity showed 0.938 of AUC (area under curve).
Group A Streptococcus (GAS) is the most common cause of bacterial pharyngitis. Streptococcal pharyngitis is characterized by an acute onset, high fever, exudative tonsillar discharge, tenderness of enlarged cervical lymph nodes, pharyngeal injection, and the absence of common cold symptoms such as coughing, sneezing, and rhinorrhea [123]. Prevalence of bacterial pharyngitis ranges from 20% to 30% in children 5–15 years old during winter season [34]. The modified Centor score is widely used to clinically analyze acute pharyngitis [124]. However, differentiation between bacterial and viral pharyngitis based solely on clinical manifestations is challenging [456].
Antibiotic treatment for GAS pharyngitis may relieve the symptoms, prevent transmission to family members and classmates, and prevent suppurative and non-suppurative complications [147]. To ensure proper antibiotic treatment and prevent antibiotic overuse or misuse, a throat culture or rapid antigen detection test (RADT) has been recommended for suspected bacterial pharyngitis [56]. In particular, RADTs are recommended as a screening test for children with acute pharyngitis [78]. RADTs have the advantage of rapid turnaround time and ease of use and do not require a bacterial culture facility. However, meta-analyses have shown that their sensitivity is approximately 86% [89]; hence, a back-up throat culture might be needed [13610]. An existing RADT, SD Bioline Strep A (SD, Yongin, Korea), has a higher sensitivity of 95.9% [11], but there is some debate regarding the need for a back-up throat culture for children with suspected bacterial pharyngitis who show a negative RADT result [2912].
Although throat culture is regarded as the gold standard for diagnosis of bacterial pharyngitis (sensitivity: 90–95% [13]), it requires microbiological expertise [14]. Culture yield is largely dependent on streaking on blood agar plates and colony isolation skill. Moreover, it takes at least one to two days to obtain the final result, which makes it difficult to prescribe antibiotics in time. Antibiotics are less appropriately prescribed when throat culture alone is used than when both throat culture and RADTs are used to diagnose bacterial pharyngitis [15].
A sensitive, accurate, and easy to use RADT is needed. While the currently available SD Bioline Strep A uses the antigen extraction solution and buffer separately [11], a recently developed RADT, SD Bioline Strep A Ultra (SD), contains these in the same tube, which facilitates its use in clinics and laboratories. In addition, SD Bioline Strep A is a strip-type test, while SD Bioline Strep A Ultra is a semi-quantitative device-type test that provides a color intensity chart representing the bacterial concentration in the pharynx. Thus, a more objective interpretation is possible in SD Bioline Strep A Ultra. We conducted a clinical evaluation of SD Bioline Strep A Ultra in children with acute pharyngitis.
Throat swabs were taken from 349 pharyngitis patients from April through September 2017 at seven pediatric clinics in Changwon, Korea. Three hundred and forty-three children were included, and six adults were excluded. Of the children, 167 (48.7%) were boys, and the mean age was 5.4 years (standard deviation 2.6 years, range 1–17 years). This study was approved by the Institutional Review Board (no. 2016-10-013) of Gyeongsang National University Changwon Hospital (GNUCH). Written informed consent was obtained from the children's parents or guardians.
Three throat swabs were collected from each child. For SD Bioline Strep A Ultra A, a flocked swab (FLOQSwabs, Copan Diagnostics Inc., Murrieta, CA, USA) was used. For SD Bioline Strep A and throat culture, two wooden cotton swabs were obtained and inserted into Amies transport medium (AM608-2S; Asan Pharmaceutical, Hwasung, Korea). Five clinics used the flocked swab first, while the other two clinics used the cotton swabs first. Transport media containing the two cotton swabs were stored at 4℃ until delivery to GNUCH (approximately 1–3 days). SD Bioline Strep A Ultra was performed by the laboratory technicians or pediatricians in the clinics, according to the manufacturer's instructions. The test results and the color intensity of the positive tests were recorded. The color intensity ranged from 1 to 20 and was read manually. A positive result was defined as a color intensity ≥1. The throat culture positive rate was evaluated according to four arbitrarily categorized color intensity ranges of SD Bioline Strep A Ultra: 1–5, 6–10, 11–15, and 16–20. SD Bioline Strep A (as described by Kim [11]) and throat culture were performed as soon as the transport tubes were delivered to GNUCH.
The swabs were inoculated on blood agar plates, and a bacitracin disk was placed on the primary streaking area. After the plates were incubated for 18–24 hours at 37℃, small, wide, β-hemolytic colonies were subjected to latex agglutination with a Seroiden Strepto kit (Eiken Co., Tokyo, Japan). Inhibition surrounding the bacitracin disk was recorded, and the number of colony forming units (CFUs) was classified into four categories: 1–10 CFU, 11–50 CFU, 51–100 CFU, and >100 CFU or pure culture. Positive rates of SD Bioline Strep A Ultra were compared with the number of CFUs. The SD Bioline Strep A Ultra results were unknown until the end of the study.
In cases of discrepancy between SD Bioline Strep A and throat culture results, the frozen-stored cotton swabs used for throat culture were tested for the speB gene to confirm GAS, using the AccuPower Taq PCR Premix (Bioneer, Daejeon, Korea). Unfortunately, not all samples with discrepant results had been stored. The speB gene was PCR amplified (forward primer, 5′-GGTTCTGCAGGTAGCTCTCG-3′; reverse primer, 5′-TGCCTACAACAGCACTTTGG-3′) with the following PCR cycling conditions: 5 minutes inactivation at 95℃, followed by 35 cycles of amplification at 95℃ for 30 seconds, 58℃ for 30 seconds, and 72℃ for 45 seconds, and a final extension at 72℃ for 7 minutes [16]. A clinical isolate of GAS was used as the positive control, and distilled water was used as the negative control.
To determine the accuracy of SD Bioline Strep A Ultra and SD Bioline Strep A, we calculated their sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with 95% confidence intervals (CIs). To estimate the diagnostic accuracy of SD Bioline Strep A Ultra, we generated an ROC curve and calculated the area under the ROC curve (AUC). The positive rate of the two RADTs was compared with the number of CFUs. In addition, the throat culture positive rates according to the four-color intensity categories (1–5, 6–10, 11–15, and 16–20) of SD Bioline Strep A Ultra were calculated, and their proportions were compared using the Fisher exact test. Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria). P<0.05 (two-tailed) was considered statistically significant.
The SD Bioline Strep A Ultra, SD Bioline Strep A, and throat culture positive rates were 58.3% (N=200), 55.7% (N=191), and 55.7% (N=191), respectively (Table 1). Of the 200 children that tested positive with SD Bioline Strep A Ultra, 54 were four years old, 39 were five years old, 33 were six years old, and 22 were three years old. Only eight were over nine years old. All GAS isolates were susceptible to bacitracin. Other groups of β-hemolytic streptococci were not isolated.
Sensitivity, specificity, PPV, and NPV (95% CI) were 97.4% (94.0–99.1%), 90.8% (85.0–94.9%), 93.0% (88.5–96.1%), and 96.5% (92.0–98.9%), respectively, for SD Bioline Strep A Ultra. Sensitivity, specificity, PPV, and NPV (95% CI) were 95.8% (91.9–98.2%), 94.7% (88.9–97.7%), 95.8% (91.9–98.2%), and 94.7% (90.0–97.7%), respectively, for SD Bioline Strep A.
Seven samples showing discrepant results between SD Bioline Strep A Ultra and throat culture were confirmed by PCR. All three samples that were SD Bioline Strep A Ultra-positive and culture-negative were positive for PCR amplification of the speB gene. All four samples that were SD Bioline Strep A Ultra-negative and culture-positive were positive by PCR.
When positive rate and the number of CFUs were compared, the positive rate of SD Bioline Strep A Ultra and SD Bioline Strep A ranged from 85.7 to 100% (P=0.021) and 78.6 to 98.3% (P=0.015), respectively (Table 2).
In this clinical evaluation of SD Bioline Strep A Ultra, the high prevalence (>50%) of GAS may have improved the accuracy of both RADTs. Their sensitivities were much higher than the average (86%) reported previously [89].
Confirmation of the discrepant results with speB PCR indicated that SD Bioline Strep A Ultra-positive and culture-negative samples were true positive, not false positive. GAS may fail to be isolated by throat culture, which raises the question of whether throat culture should be regarded as the gold standard, in comparison with a more sensitive detection method [13]. An SD Bioline Strep A Ultra-positive and culture-negative sample had a color intensity of 1, suggesting misreading of the color. Although the pediatricians and technicians were trained prior to study initiation, optimal standardization of reading the weak color may be difficult.
Most previous RADT studies were qualitative and did not analyze the number of CFUs, which might have caused differences in their results [21215]. In our study, RADT sensitivity was lower when the number of CFUs was smaller (Table 2). Similar results were observed when the inoculum effect of a single swab and two combined swabs were compared in a previous study [12].
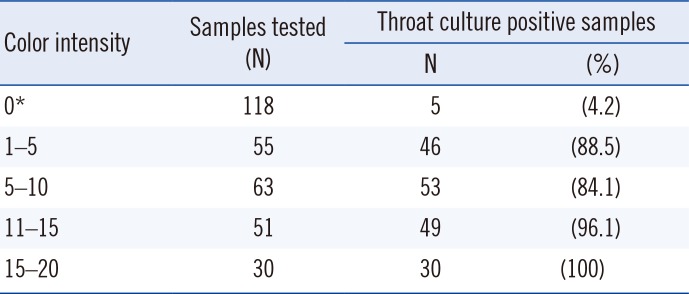
Among samples with a color intensity of 1–10, 86.1% (99/115) were positive by throat culture. In contrast, among samples with a color intensity >10, 97.5% (79/81) were positive by throat culture. Color intensity seems strongly related to GAS positivity (Table 3); future studies should investigate the usefulness of this color intensity chart.
This study had several limitations. First, we used different types of swabs. As the two cotton swabs were inserted into the same transport tube, the agreement between SD Bioline Strep A and throat culture appears better. In general, flocked swabs collect a higher number of organisms than cotton swabs do [1718]. This might have caused a slight difference in performance between the tests. Another possible limitation is that different numbers of GAS could have been obtained between the first and second swab. The sampling order or throat swab technique might have affected the results because of spectrum size and GAS distribution [1012]. In addition, as the pediatric clinics did not have a facility for growing bacteria, transport media containing cotton swabs should be delivered to GNUCH. As we collected the samples from seven clinics, delivery was delayed at times, especially during weekends and holidays. Although GAS can survive several days in the transport medium at 4℃, decay may have occurred during the delayed delivery. This factor might have caused the lower yield observed for SD Bioline Strep A and throat culture.
In conclusion, SD Bioline Strep A Ultra exhibited excellent sensitivity and NPV and performance similar to the currently used SD Bioline Strep A, in comparison with throat culture. Three of the SD Bioline Strep A Ultra-positive and culture-negative samples were positive for speB gene amplification, suggesting that these cases may be not false positive. In addition, the positive rate of SD Bioline Strep A Ultra differed by the number of CFUs of GAS.
Acknowledgment
The authors sincerely thank the pediatricians who participated in this clinical study. In addition, we wish to thank the Green Cross LabCell team for transport of the samples, Jungin Heo, MT, for her dedicated work of bacterial isolation, and Rok-bum Kim, MD, Gyeongsang National University Hospital, for his excellent statistical analysis.
References
1. Choby BA. Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009; 79:383–390. PMID: 19275067.
2. Edmonson MB, Farwell KR. Relationship between the clinical likelihood of group A streptococcal pharyngitis and the sensitivity of a rapid antigen-detection test in a pediatric practice. Pediatrics. 2005; 115:280–285. PMID: 15687433.
3. Kalra MG, Higgins KE, Perez ED. Common questions about streptococcal pharyngitis. Am Fam Phys. 2016; 94:24–31.
4. Wessels MR. Clinical practice. Streptococcal pharyngitis. N Engl J Med. 2011; 364:648–655. PMID: 21323542.
5. Gerber MA. Diagnosis and treatment of pharyngitis in children. Pediatr Clin North Am. 2005; 52:729–747. vi. PMID: 15925660.
6. Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012; 55:1279–1282. PMID: 23091044.
7. Webb KH, Needham CA, Kurtz SR. Use of a high-sensitivity rapid strep test without culture confirmation of negative results: 2 years’ experience. J Fam Pract. 2000; 49:34–38. PMID: 10678338.
8. Cohen JF, Bertille N, Cohen R, Chalumeau M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database Syst Rev. 2016; 7:CD010502. PMID: 27374000.
9. Lean WL, Arnup S, Danchin M, Steer AC. Rapid diagnostic tests for group A streptococcal pharyngitis: a meta-analysis. Pediatrics. 2014; 134:771–781. PMID: 25201792.
10. Hall MC, Kieke B, Gonzales R, Belongia EA. Spectrum bias of a rapid antigen detection test for group A β-hemolytic streptococcal pharyngitis in a pediatric population. Pediatrics. 2004; 114:182–186. PMID: 15231926.
11. Kim S. The evaluation of SD Bioline Strep A rapid antigen test in acute pharyngitis in pediatric clinics. Korean J Lab Med. 2009; 29:320–323. PMID: 19726894.
12. Kurtz B, Kurtz M, Roe M, Todd J. Importance of inoculum size and sampling effect in rapid antigen detection for diagnosis of Streptococcus pyogenes pharyngitis. J Clin Microbiol. 2000; 38:279–281. PMID: 10618101.
13. Gieseker KE, Mackenzie T, Roe MH, Todd JK. Comparison of two rapid Streptococcus pyogenes diagnostic tests with a rigorous culture standard. Pediatr Infect Dis J. 2002; 21:922–927. PMID: 12394813.
14. Bisno AL. Acute pharyngitis. N Engl J Med. 2001; 344:205–211. PMID: 11172144.
15. Lieu TA, Fleisher GR, Schwartz JS. Clinical evaluation of a latex agglutination test for streptococcal pharyngitis: performance and impact on treatment rates. Pediatr Infect Dis J. 1988; 7:847–854. PMID: 3062561.
16. Dunne EM, Marshall JL, Baker CA, Manning J, Gonis G, Danchin MH, et al. Detection of group A streptococcal pharyngitis by quantitative PCR. BMC Infect Dis. 2013; 13:312. PMID: 23844865.
17. Hernes SS, Quarsten H, Hagen E, Lyngroth AL, Pripp AH, Bjorvatn B, et al. Swabbing for respiratory viral infections in older patients: a comparison of rayon and nylon flocked swabs. Eur J Clin Microbiol Infect Dis. 2011; 30:159–165. PMID: 20853014.
18. Dube FS, Kaba M, Whittaker E, Zar HJ, Nicol MP. Detection of Streptococcus pneumoniae from different types of nasopharyngeal swabs in children. PLoS One. 2013; 8:e68097. PMID: 23840817.
Fig. 1
ROC plot for color intensity of SD Bioline Ultra test.
Abbreviation: CI, confidence interval.
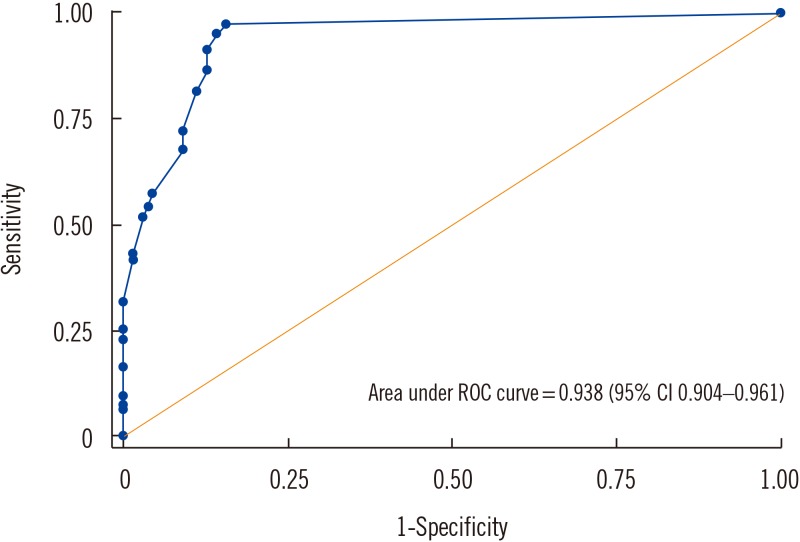
Table 1
Comparison of SD Bioline Strep A Ultra and SD Bioline Strep A results versus throat culture
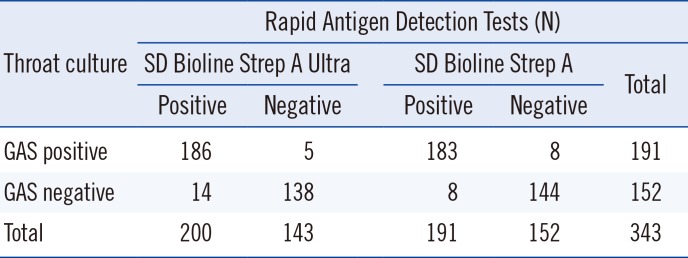
Table 2
Positive rate of SD Bioline Strep A Ultra and SD Bioline Strep A in comparison with the number of CFUs
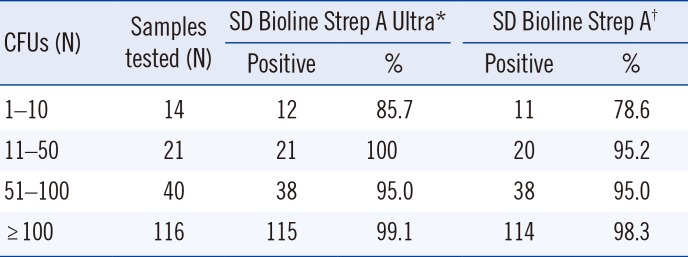
| CFUs (N) | Samples tested (N) | SD Bioline Strep A Ultra* | SD Bioline Strep A† | ||
|---|---|---|---|---|---|
| Positive | % | Positive | % | ||
| 1–10 | 14 | 12 | 85.7 | 11 | 78.6 |
| 11–50 | 21 | 21 | 100 | 20 | 95.2 |
| 51–100 | 40 | 38 | 95.0 | 38 | 95.0 |
| ≥100 | 116 | 115 | 99.1 | 114 | 98.3 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download