Abstract
Background
Cutaneous leishmaniasis is a tropical infection of public health importance. Numerous treatment approaches are in practice with variable degree of success however its management has no universal consensus or practice guidelines to follow.
Objective
Analyze the management of cutaneous leishmaniasis retrospectively at a central hospital of Jazan Province, Kingdom of Saudi Arabia to identify the current treatment pattern and compare the outcomes.
Methods
This cross-sectional study was conducted based on the hospital records of patients who attended the dermatology clinic for cutaneous leishmaniasis during the year 2012 to 2015.
Results
Forty three patients were included in the study. There was a male preponderance (65.1%) among the patients and 60.5% of them were of pediatric age group. Monotherapy was the initial choice for 58.1% of the patients. Intralesional sodium stibogluconate (SS-IL) was the most preferred treatment for initial therapy, as monotherapy and as part of combination therapy. A complete response was achieved in 22 patients (51.2%) with initial therapy. Among the different treatment groups, SS-IL+itraconazole showed significantly higher complete response rate compared to other treatments offered as initial therapy (p<0.01). Initial SS-IL monotherapy provided complete response in 41.2% patients receiving it, while itraconazole monotherapy provided complete response in 75% and 90.9% of the patients receiving initial itraconazole+SS-IL combination therapy with achieved complete response.
Leishmaniasis is a major public health issue prevalent across the world and endemic in several countries resulting in significant morbidity and mortality. It is a parasitic disease caused by a protozoan belonging to the genus Leishmania and known to be transmitted by the bite of infected female sandflies. It can present as cutaneous, mucocutaneous or visceral leishmaniasis1.
Cutaneous leishmaniasis (CL) is the most common and least fatal form of the disease, typically presenting with ulcerative skin lesions usually on the uncovered areas of the body such as face, forearms and lower legs2.
The number of CL patients has increased in the last few decades mainly due to human migration between non-endemic and endemic areas, and adaptation of the Leishmania parasites to additional vectors and mammalian hosts34. Majority of all CL cases occur in only seven countries: Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria5. The Middle Eastern region is known to be endemic for CL (18 out of 23 countries) and also several reports come from this region due to vast number of immigrants for work3. The CL which is wide spread in the Middle-East is distinguished as ‘Old-World’ type, which is due to L. major, L. tropica, and L. infantum6.
CL is endemic in some parts of the Kingdom of Saudi Arabia (KSA) including parts of Jazan Province7. L. major is the most important species causing CL in KSA while L. tropica is also known to cause cutaneous disease in southwestern parts of KSA8. Phlebotomus sergenti is the main vector for leishmania in KSA while desert rodents like gerbils and desert rats are the primary reservoirs2. The skin lesions produced by L. major tend to be large and wet, while L. tropica often causes dry lesions with a central crust9. As per World Health Organization report, 4,753 cases were reported in 2006 from KSA, and by the year 2012 the prevalence declined, with nearly 1,400 cases being reported10.
Confirmation of leishmaniasis is done by identification of amastigotes using Giemsa stain of biopsy tissue or aspirate from lesions or by culture using Novy, MacNeal, and Nicolle (biphasic) medium. The leishmania species identification can be done with polymerase chain reaction (PCR). The leishmanin skin test may be useful in some patients with CL11.
Parenteral stibogluconate has been the treatment of choice for CL since last 70 years, but it is associated with poor patient compliance and serious toxicities (renal, hepatic and cardiac) and hence there has been extensive search for other treatment approaches12. Sodium stibogluconate intralesional (SS-IL) is currently being used in hospitals in KSA as standard of care in CL. However the treatment schedules with SS-IL varies with hospitals. Once-weekly, twice-weekly, alternate day and daily regimens are in practice1314. Topical 15% paromomycin15, antifungals7, topical liposomal amphotericin B16, topical radio-frequency-induced heat therapy17, laser therapy18, photodynamic therapy19 and cryotherapy have shown variable efficacy against various species causing CL including L. major.
Combination approach was found to be better than monotherapy in many studies. SS-IL in combination with oral ketoconazole20 and topical paromomycin in combination with intralesional meglumine antimoniate were shown to be effective21.
With the introduction of newer drugs with fewer side effects and modified dosing schedules, alternative options or improvised treatment strategies have been adopted in the treatment of CL. However, there is no consensus yet in the drug of choice, optimal dose or duration of treatment22.
The current observational study retrospectively evaluated various therapeutic interventions adopted in the management of CL cases during the year 2012 to 2015 at a central hospital in Jazan Province and compared the outcomes associated with various types of treatment practices to identify the treatment approaches with best outcomes among them.
A cross-sectional study design was utilized using the hospital records of patients attending the dermatology clinic for CL in tertiary care centre King Fahd Central Hospital (KFCH) in Jazan Province. The study sample included all the CL patients who were treated at the dermatology clinic of KFCH during the period of four years from January 2012 to December 2015. Of this sample, the patients with a parasitologically (biopsy, stain, culture or PCR) confirmed diagnosis of CL within the study period (2012 to 2015) were included in the study. Patients with incomplete medical records were excluded from the study. The final sample size of the study was 43. The study was approved by the Standing Committee for Biomedical Ethics of Jazan University (IRB no. 1436-SCBRE-23).
A case record form (CRF) was developed and was used for collection of demographic characteristics, clinical data, and treatment information. The information regarding management of CL in the patients eligible to be included in the study were obtained from medical records department. Each patient case file was discussed with the dermatology consultants and accuracy of the information available on the files was verified. Anonymity of the study subjects were maintained in the CRF by using code names.
The CRF of the 43 patients included in the study period were reviewed and entered into the data management system. Data management and analysis was carried out using IBM SPSS version 22 (IBM Co., Armonk, NY, USA). The participants were grouped based on the medications used for treatment. Complete descriptive analyses of all variables were performed and have been presented as number and percentages and mean. Inferential statistics were carried out for categorical variables using Chi-square and Fisher's exact test as appropriate. Two sided p-values less than 0.05 were considered significant.
A total of 43 patients were included in the study and their management was analyzed in detail. A male preponderance (65.1%) was observed in the sample and also 26 (60.5%) of sample were of pediatric age. The average age of the subjects was 17.1±14.13 years with a minimum and maximum age of 1 and 64 years respectively. The details of demographic and clinical characteristics of the study population have been listed in Table 1.
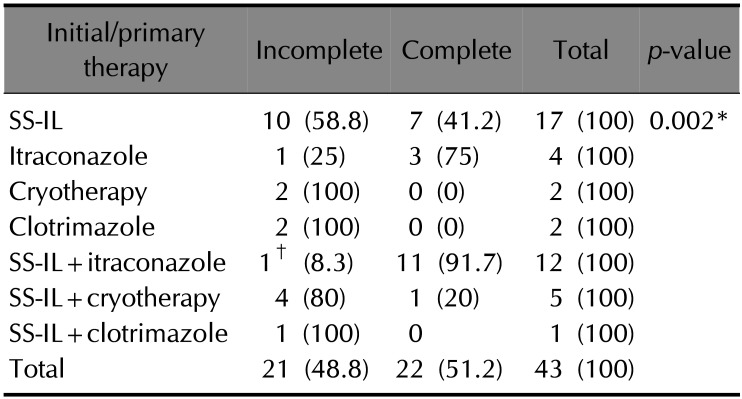
Among the 43 patients, 25 (58.1%) of them received initial monotherapy while 18 (41.9%) of them received initial combination therapy. SS-IL was the most preferred initial treatment approach and 35 (81.4%) patients received it as initial therapy. Among the patients receiving initial therapy, 17 (39.5%) received SS-IL as monotherapy and 18 (41.9%) received it as part of combination therapy with itraconazole (n=12), cryotherapy (n=5) or clotrimazole (n=1). Itraconazole was the second most favored drug for initial therapy with 4 patients receiving it as monotherapy and 12 receiving it as part of combination therapy.
A complete response was achieved in 22 patients (51.2%) out of 43 patients with initial therapy itself, while remaining 21 patients needed additional therapy to achieve complete cure. SS-IL provided complete response with monotherapy in 41.2% patients receiving the drug and 66.7% among those on combination therapy containing SS-IL achieved complete response. Itraconazole monotherapy provided complete response in 75% of the patients receiving it, while 90.9% of the patients on initial therapy with SS-IL+itraconazole combination had complete response.
On comparing the response rates among the different treatment groups (Table 2), SS-IL+itraconazole showed significantly higher complete response rates compared to other treatment groups that showed complete response (p<0.01). There was no significant difference in the duration of initial therapy among patients receiving monotherapy and combination therapy.
Additional therapy was required for 21 patients and 18 of them achieved complete cure. Itraconazole was found to be the most favored drug as an additional treatment option. Among the patients requiring additional treatment, 66.7% (14 patients) of them received itraconazole, either as monotherapy (12 patients) or as combination therapy with SS-IL (2 patients) and all of them achieved complete response (Table 3).
A total of 3 patients failed to respond to the additional therapy and those 3 patients subsequently responded with complete cure following treatment with itraconazole.
Most commonly reported side effect was mild injection site inflammation and pain with SS-IL injections, but none of the patients required discontinuation of the treatment. One patient on itraconazole along with SS-IL had elevated liver enzymes which necessitated change in the medication. SS-IL was most commonly used medication as initial therapy. All the patients treated with SS-IL received lignocaine along with the stibogluconate to minimize pain and improve patient compliance. Stibogluconate was used in 1:1 ratio with lignocaine and 1~2 ml of the mixture was used for once weekly intralesional injections according to the size and location of the lesion. Facial lesions and smaller lesions were mostly injected with 1 ml of the mixture (0.5 ml of 1% lignocaine with 0.5 ml of stibogluconate) while larger lesions were injected with 2 ml or more depending on the size of the lesion. The duration of the treatment ranged from 8 to 18 weeks based on the treatment response.
Itraconazole was used at dose of 200 mg twice daily for 6~12 weeks in patients above 12 years and younger patients were given 100 mg twice daily for 6 weeks. Children below 5 years of age and those below 20 kg body weight were given itraconazole oral solution 5 mg/kg/day in 2 divided doses. A 6 week therapy was more frequently observed in the patients receiving combination therapy.
Clotrimazole cream was used topically twice daily in a few patients but the approach showed low response rates and the approach was less favored among the dermatologists. All patients with open lesions and patients receiving cryotherapy were additionally given 2% fusidic acid cream for local application to prevent any secondary bacterial infection.
CL is the most common form of leishmaniasis present in KSA. Long term epidemiological reports have shown gradual decline in the number of cases being reported from KSA. The success is attributed to the active national control programs, detecting cases in focus areas, vector control programs and reservoir control programs to further control insect and non-human reservoirs2. The disease in KSA is mostly known to affect individuals of 15~44 years of age and generally affects extremities; most patients have a single lesion, with less than 5% showing multiple lesions on hands, legs, and face2.
The main objective of our study was to provide a comprehensive overview of the CL and evaluation of the treatment in Jazan Province of KSA. In our study, the pediatric population 26 (60.5%) was more commonly affected by CL compared to the adults probably due to their outdoor games and activities, particularly in the hilly regions of Jazan. This is not fully in agreement with previous studies from KSA2 which reported that most patients were of 15~44 years of age and with previous studies conducted in Sri Lanka and in India where adults were at a higher risk of exposure due to occupation related outdoor activities423.
We observed CL infection was higher among males than females. This is consistent with previous studies reported from Sri Lanka, Libya, Pakistan, and Iran24. This observation could be because males are more likely to work or play in open environments and also that the women in KSA are protected by the covering garments.
About 79% of the patients in this study group had single lesion. Similar lesion pattern was reported in previous studies conducted in KSA and in countries such as Sri Lanka, India and Tunisia472325. In the present study, lesions were more common in the head and neck region, unlike reports of Galgamuwa et al.4 wherein upper limb was the common site and Salam et al.2 who reported most lesions on the extremities. The face was commonly involved in CL lesions in studies conducted in other countries like India23. These findings are in general agreement with the fact that exposed parts of the body are more prone for sandfly bite.
In the present study, SS-IL was found to be the most commonly prescribed drug for initial treatment, as monotherapy and as combination therapy. There were no reports of compliance issues with SS-IL therapy which is often associated with injection site pain. This is mostly due to the use of lignocaine along with the intralesional injection of stibogluconate. However with reports of increasing prevalence of resistance against pentavalent antimony compounds, newer treatment approaches were observed to be used more frequently. Many physicians were increasingly opting for use of initial combination therapy containing pentavalent antimony compounds to minimize resistance and to improve response rates. Among the different treatment groups, SS-IL+itraconazole combination therapy showed significantly higher complete response rates compared to other treatment groups that showed complete response (p<0.01) while other combination therapy approaches were not giving satisfactory rates of complete response. Initial itraconazole monotherapy provided complete response in 75% of the patients receiving it, while 90.9% of those receiving it as combination therapy achieved complete response.
Significant regional variation in efficacy is observed with different treatment approaches evaluated globally. SS-IL therapy has reported high response rates in various studies. Monotherapy with various antifungals including itraconazole and fluconazole in previous studies from other parts of KSA documented cure rate of 70%~79% with six week course of fluconazole at a dose of 200 mg/day and 65% with itraconazole 150 mg/day for CL patients726. A randomized control study in Egypt reported SS-IL in combination with oral ketoconazole to be more effective than SS-IL alone20. However no studies using itraconazole combination as initial choice of therapy for management of CL have been reported in the region.
In the present study there was no significant difference in the duration of the treatment among the treatment groups receiving initial therapy. However, the patients with incomplete response to initial therapy had to receive additional treatments which prolonged the total duration of therapy. Itraconazole was the most common choice for additional therapy and all patients receiving it achieved complete response.
A specific criterion for choice of therapy was not observed but a clear pattern of increasing use of combination therapy using SS-IL with itraconazole as initial therapy and itraconazole monotherapy as additional therapy was observed towards the latter part of the study period. This observation points to the increasing acceptance of the efficacy of itraconazole based therapy among the dermatologists in the region.
In our study only one patient had documented serious side effect requiring treatment change. A patient who was on itraconazole treatment along with SS-IL was associated with elevated liver enzymes after 6 weeks of therapy and the treatment was replaced with topical miconazole.
The local application of 2% fusidic acid in patients with open lesions may have contributed in prevention of secondary infections and in faster healing of the open lesions. Some patients without open lesions, receiving SS-IL also received fusidic acid cream for topical application however it is doubtful if such a practice is of benefit to the patients.
A questionnaire survey among physicians treating CL from KSA had reported disparities in the type and duration of treatment and had suggested need for guidelines for treatment of CL and a nationwide policy considering all factors that affect the outcome of treatment22. Our study was a single centre study and the relatively smaller sample size limits the generalization of the results to the entire country or region. Extending the study to other hospitals and health centres across the province and different provinces in the country can be valuable in the formulation of guidelines and nationwide policies in management of CL.
In conclusion, the study finds that adopting combination therapy with SS-IL+itraconazole as the initial treatment for CL can safely improve complete response rates and reduce the need for additional or prolonged therapies. However, additional studies with larger patient populations including those from other regions of the Kingdom are required for generalization of the results. The other findings and observations were reasonably in agreement with earlier reports from other parts of KSA and that of other endemic countries.
ACKNOWLEDGMENT
The authors would like to express sincere thanks to Dr. Mohammed Al Bratty, Mr Sarfaraz Ahmed and Mr Omer Siddiq for all their support and KFCH medical director for permitting the conduct of study and providing access to the medical records of the patients. The authors would like to thank Medical Research Center of Jazan University for funding the study.
References
1. Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005; 366:1561–1577. PMID: 16257344.

2. Salam N, Al-Shaqha WM, Azzi A. Leishmaniasis in the middle east: incidence and epidemiology. PLoS Negl Trop Dis. 2014; 8:e3208. PMID: 25275483.

3. Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007; 7:581–596. PMID: 17714672.

4. Galgamuwa LS, Sumanasena B, Yatawara L, Wickramasinghe S, Iddawela D. Clinico-epidemiological patterns of cutaneous leishmaniasis patients attending the Anuradhapura Teaching Hospital, Sri Lanka. Korean J Parasitol. 2017; 55:1–7. PMID: 28285499.

5. Hepburn NC. Cutaneous leishmaniasis: current and future management. Expert Rev Anti Infect Ther. 2003; 1:563–570. PMID: 15482153.

6. Masmoudi A, Hariz W, Marrekchi S, Amouri M, Turki H. Old world cutaneous leishmaniasis: diagnosis and treatment. J Dermatol Case Rep. 2013; 30:31–41.

7. Khan W, Zakai HA. Epidemiology, pathology and treatment of cutaneous leishmaniasis in taif region of Saudi Arabia. Iran J Parasitol. 2014; 9:365–373. PMID: 25678921.
8. al-Zahrani MA, Peters W, Evans DA, Smith V, Ching Chin I. Leishmania infecting man and wild animals in Saudi Arabia. 6. Cutaneous leishmaniasis of man in the south-west. Trans R Soc Trop Med Hyg. 1989; 83:621–628. PMID: 2617623.

9. Aoun K, Bouratbine A. Cutaneous leishmaniasis in North Africa: a review. Parasite. 2014; 21:14. PMID: 24626301.

10. World Health Organization. Leishmaniasis [Internet]. Geneva: World Health Organization;2012. cited 2014 Dec 17. Available from: http://www.who.int/leishmaniasis/burden/en/.
11. Rodríguez N, Guzman B, Rodas A, Takiff H, Bloom BR, Convit J. Diagnosis of cutaneous leishmaniasis and species discrimination of parasites by PCR and hybridization. J Clin Microbiol. 1994; 32:2246–2252. PMID: 7814554.

12. Blum J, Desjeux P, Schwartz E, Beck B, Hatz C. Treatment of cutaneous leishmaniasis among travellers. J Antimicrob Chemother. 2004; 53:158–166. PMID: 14729756.

13. Faris RM, Jarallah JS, Khoja TA, Al-Yamani MJ. Intralesional treatment of cutaneous leishmaniasis with sodium stibogluconate antimony. Int J Dermatol. 1993; 32:610–612. PMID: 8407083.

14. Tallab TM, Bahamdam KA, Mirdad S, Johargi H, Mourad MM, Ibrahim K, et al. Cutaneous leishmaniasis: schedules for intralesional treatment with sodium stibogluconate. Int J Dermatol. 1996; 35:594–597. PMID: 8854166.

15. Asilian A, Jalayer T, Nilforooshzadeh M, Ghassemi RL, Peto R, Wayling S, et al. Treatment of cutaneous leishmaniasis with aminosidine (paromomycin) ointment: double-blind, randomized trial in the Islamic Republic of Iran. Bull World Health Organ. 2003; 81:353–359. PMID: 12856053.
16. Layegh P, Rajabi O, Jafari MR, Emamgholi Tabar, Moghiman T, Ashraf H, et al. Efficacy of topical liposomal amphotericin B versus intralesional meglumine antimoniate (Glucantime) in the treatment of cutaneous leishmaniasis. J Parasitol Res. 2011; 2011:656523. PMID: 22174993.

17. Bumb RA, Prasad N, Khandelwal K, Aara N, Mehta RD, Ghiya BC, et al. Long-term efficacy of single-dose radiofrequency-induced heat therapy vs. intralesional antimonials for cutaneous leishmaniasis in India. Br J Dermatol. 2013; 168:1114–1119. PMID: 23298394.

18. Asilian A, Sharif A, Faghihi G, Enshaeieh Sh, Shariati F, Siadat AH. Evaluation of CO laser efficacy in the treatment of cutaneous leishmaniasis. Int J Dermatol. 2004; 43:736–738. PMID: 15485530.
19. Gardlo K, Horska Z, Enk CD, Rauch L, Megahed M, Ruzicka T, et al. Treatment of cutaneous leishmaniasis by photodynamic therapy. J Am Acad Dermatol. 2003; 48:893–896. PMID: 12789181.

20. El-Sayed M, Anwar AE. Intralesional sodium stibogluconate alone or its combination with either intramuscular sodium stibogluconate or oral ketoconazole in the treatment of localized cutaneous leishmaniasis: a comparative study. J Eur Acad Dermatol Venereol. 2010; 24:335–340. PMID: 19744259.

21. Moosavi Z, Nakhli A, Rassaii S. Comparing the efficiency of topical paromomycine with intralesional meglumine antimoniate for cutaneous leishmaniasis. Int J Dermatol. 2005; 44:1064–1065. PMID: 16409282.
22. Al-Jaser MH. Treatment trends of cutaneous leishmaniasis in Saudi Arabia. Saudi Med J. 2005; 26:1220–1224. PMID: 16127517.
23. Sharma NL, Mahajan VK, Kanga A, Sood A, Katoch VM, Mauricio I, et al. Localized cutaneous leishmaniasis due to leishmania donovani and leishmania tropica: preliminary findings of the study of 161 new cases from a new endemic focus in himachal pradesh, India. Am J Trop Med Hyg. 2005; 72:819–824. PMID: 15964970.

24. Feiz-Haddad MH, Kassiri H, Kasiri N, Panahandeh A, Lotfi M. Prevalence and epidemiologic profile of acute cutaneous leishmaniasis in an endemic focus, Southwestern Iran. J Acute Dis. 2015; 4:292–297.

25. Zaraa I, Ishak F, Kort R, El Euch D, Mokni M, Chaker E, et al. Childhood and adult cutaneous leishmaniasis in Tunisia. Int J Dermatol. 2010; 49:790–793. PMID: 20618499.

26. Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by leishmania major. N Engl J Med. 2002; 346:891–895. PMID: 11907288.
Table 1
Details of characteristics of the patients (n=43)
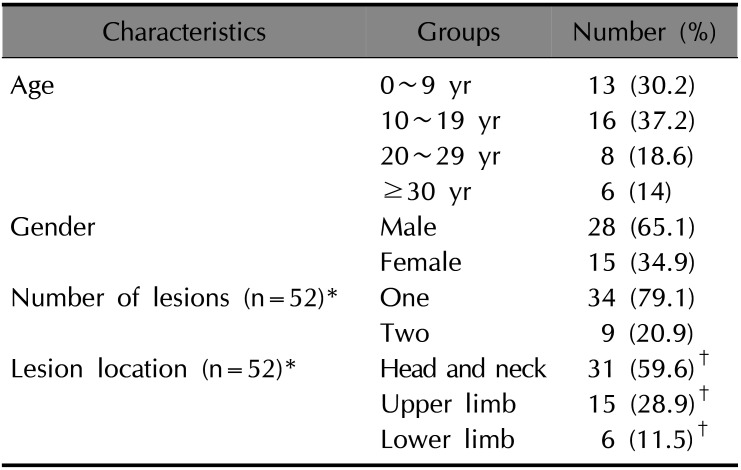
Table 2
Response with initial therapy across treatment groups (row %)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download