Abstract
Background
Controlling inflammation is a therapeutic goal of various autoimmune/autoinflammatory diseases including Behçet's disease (BD). The immunomodulatory effect of metabolites or metabolic analogs such as butyrate and 3-bromopyruvate has been observed in animal disease models.
Objective
We attempted to evaluate the effect of butyrate and 3-bromopyruvate on the inflammatory cytokine production by peripheral blood mononuclear cells (PBMCs) isolated from patients with mucocutaneous involvement of BD.
Methods
PBMCs isolated from 11 patients with BD and 10 healthy controls were stimulated with lipopolysaccharide in the presence of butyrate or 3-bromopyruvate. Butyrate receptor and cytokine messenger ribonucleic acid (mRNA) expression was analyzed by real-time reverse transcription polymerase chain reaction. Cytokine secretion was assessed by enzyme-linked immunosorbent assay. PBMCs survival was analyzed by flow cytometry.
Results
Bromopyruvate or butyrate treatment suppressed inflammatory cytokine production in PBMCs from all our subjects. Bromopyruvate also reduced PBMCs survival while butyrate did not. As the effect of butyrate was slightly greater in BD patients than in healthy controls, we analyzed butyrate receptor expression and found that lipopolysaccharide-induced free fatty acid receptor 2 mRNA level in PBMCs was higher in BD patients than in controls.
Behçet's disease (BD) is a chronic inflammatory disease in multi-organs1. The microbial agents or autoantigens are proposed to trigger inflammation in genetically susceptible individuals1. Immunosuppressive agents including anti-tumor necrosis factor (TNF)-α antibody are recommended when the first line drugs such as colchicine and corticosteroid are ineffective1. The goal of BD treatment is reducing the severity and frequency of inflammatory attacks in order to prevent irreversible organ damage1.
A prospective target for controlling inflammation is glycolysis, a pivotal metabolic pathway used by immune cell subtypes to produce inflammatory cytokine2. Bromopyruvate (BrPA) inhibits glycolysis through targeting hexokinase 2 and glyceraldehyde 3-phosphate dehydrogenase3. Therefore, BrPA ameliorates disease severity in a multiple sclerosis mouse model3 and inhibits arthritis progression in a mouse model4.
Another metabolic regulator, short chain fatty acids (SCFAs) (e.g., butyrate) affect glucose and lipid metabolism5 and modify gene expression through interacting with their receptors and regulating histone modifying enzymes6. SCFAs are supplied to humans mainly by colonic anaerobic bacteria fermenting undigested saccharides and dietary fibers5. A recent report presents a significant decrease in butyrate production and dysbiosis in the guts of patients with BD7. However, the effect of SCFA or BrPA on the inflammatory cytokine production in peripheral blood mononuclear cells (PBMCs) of patients with BD has not been reported.
Patients diagnosed with BD according to the International Study Group and Japanese criteria for BD in the Department of Dermatology at the Ajou University Hospital were enrolled in this study. We recruited a total of 21 subjects across three groups: five patients with active BD (two were male, mean age±standard deviation [SD]=44.2±9.0), six patients with inactive BD (four were male, mean age±SD=44.5±12.2) and ten healthy controls (HC; three were male, mean age±SD=37.3±8.2). No statistically significant difference was found in the male to female ratio among the groups (p>0.05, chi-squared test). Patients in the active BD group had at least one BD symptom despite receiving treatment, and those in the inactive group had no symptoms due to anti-inflammatory medications (Table 1). The metabolic status of patients would affect the results, given that bromopyruvate and butyrate inhibit metabolic enzymes. However, we did not exclude any subjects due to difficulty recruiting BD patients. Metabolic disease (diabetes mellitus or hypothyroidism) was found in two patients with inactive BD. The study was approved by the Ajou University Hospital Institutional Review Board (IRB no. AJIRB-BMR-GEN-14-463) and informed consent was obtained from all subjects.
THP-1 cells were cultured in RPMI1640 (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (Capricorn Scientific, Auf der Lette, Germany), penicillin (100 U/ml, Thermo Fisher Scientific), and streptomycin (100 µg/ml, Thermo Fisher Scientific). PBMCs were isolated using Ficoll-Paque Plus (GE Healthcare, Chicago, IL, USA). THP-1 cells (1×106/ml) were stimulated with 100 ng/ml lipopolysaccharide (LPS) (Sigma-Aldrich, St Louis, MO, USA) in the presence or absence of various concentrations of bromopyruvate (Sigma-Aldrich) or butyrate (Sigma-Aldrich). PBMCs (1×106/ml) were stimulated with 100 ng/ml LPS in the presence or absence of 80 µM of bromopyruvate or 10 mM of butyrate.
Total RNA was extracted from THP-1 cells or PBMCs with RNAiso (Takara Bio, Otsu, Japan) and reverse transcribed using PrimeScript RTase (Takara Bio). Real-time RT-PCR was done using SYBR gene Ex Taq Premix (Takara Bio) and specific primers for interleukin (IL)-1β, IL-6, TNF-α free fatty acid receptor 2 (FFAR2), FFAR3 and actin (Takara Bio). PCR was run on a Quant Studio 3 (Applied Biosystems, Foster City, CA, USA). The cytokine or FFAR messenger ribonucleic acid (mRNA) amount was normalized to the actin mRNA level. The data was presented as relative mRNA level at one time point of culture to the mRNA level at the beginning of culture.
Cytokine levels in culture supernatant were assessed by ELISA using IL-1β ELISA kit (R&D systems, Minneapolis, MN, USA) and IL-6 and TNF-α ELISA kit (Biolegend, San Diego, CA, USA).
Cells were labeled with propidium iodide (Merck Millipore, Billerica, MS, USA) in phosphate buffered saline and then analyzed with flow cytometer (FACScanto; BD Biosciences, Franklin Lakes, NJ, USA).
Kruskal-Wallis test was done to evaluate the differences among three groups (HC, active BD and inactive BD). Chi-squared test was done to analyze the gender differences among three groups. Mann-Whitney U-test was conducted to analyze the significance of the suppressive effect of drugs. A difference with a p-value<0.05 was considered significant.
We first determined effective doses of BrPA and butyrate by analyzing their effects on THP-1 cell cytokine production at 3 hours of LPS stimulation (Fig. 1). Since mRNA levels of IL-6, TNF-α and IL-1β reached their peaks when stimulated for 3 hours, we treated THP-1 cells with various concentrations of BrPA or butyrate for 3 hours. BrPA downregulated mRNA levels of all these cytokines in a concentration-dependent manner. Butyrate also reduced IL-6 mRNA levels in a concentration-dependent manner but did not reduce TNF-α or IL-1β levels. BrPA and butyrate were the most effective at their highest concentrations, 80 µM and 10 mM, respectively. Therefore, we evaluated the effect 80 µM BrPA or 10 mM butyrate across three time points (1, 3 and 6 hours of stimulation) on cytokine mRNA expression. BrPA suppressed cytokine mRNA expression at every time point, while butyrate suppressed expression only at early time points. We assessed cytotoxicity of these drugs and found that BrPA reduced cell survival while butyrate did not.
We next examined the effects of BrPA and butyrate on the cytokine expression in PBMCs isolated from BD patients as well as HC (Fig. 2). Transcript levels of IL-6, TNF-α and IL-1β in PBMCs increased and persisted throughout 6 hours of LPS stimulation. These cytokine mRNA levels were higher in BD patients than those in HC, but the protein levels were similar between groups. BrPA treatment suppressed both mRNA and protein expression of these cytokines in PBMCs of all the subjects. Notably, BrPA cytotoxicity was observed in PBMCs of all the subjects (Fig. 2G).
Butyrate treatment inhibited IL-6 mRNA and protein expression in PBMCs of all subjects at all examined time points. It also suppressed TNF-α mRNA and protein expression at 6 hours. Although it downregulated IL-1β mRNA expression only in PBMCs of BD patients, it suppressed IL-1β protein expression in all subjects at 6 hours. Notably its suppressive effect on IL-1β protein levels was twofold greater in BD patients than in HC. Yet, butyrate did not affect PBMCs survival (data not shown).
As the effect of butyrate was slightly greater in BD patients than in HC, we investigated whether the butyrate receptor mRNA level in PBMCs of BD patients was greater than that of HC (Fig. 3). Contrary to our expectation, FFAR2 but not FFAR3 mRNA expression significantly decreased in BD patients in ex vivo PBMCs compared to HC. However, when PBMCs were stimulated with LPS, FFAR2 levels relative to the average mRNA level in unstimulated HC PBMCs increased approximately twofold, six fold and sevenfold at 1 hour in HC, inactive BD and active BD patients, respectively and further increased only in BD patients after 3 hours. FFAR3 expression also increased in LPS-stimulated PBMCs of all subjects with no significant differences among groups. BrPA and butyrate treatment significantly downregulated FFAR2 expression in all subjects at all examined time points. Also, BrPA and butyrate treatment significantly downregulated FFAR3 expression at most but not all time points.
In this study, we showed BrPA treatment suppressed inflammatory cytokine expression in PBMCs of all the subjects. These results are concordant with the report that BrPA reduces IL-6 and TNF-α production by LPS-stimulated mouse dendritic cells in a concentration-dependent manner4. We also observed that BrPA increased PBMCs death frequency. Although the in vivo BrPA effect on the activated immune cell survival has not been specifically studied, cell death was anticipated in activated PBMCs given previous studies by Ko et al.8 and Lachmandas et al.9 Ko et al.8 found that low concentrations of BrPA (20~90 µM) led to cell death in approximately 90% of hepatocellular tumor cells but in approximately 20% of normal hepatocytes. Lachmandas et al.9 reported that LPS-stimulated monocytes depend on glycolysis for adenosine triphosphate (ATP) production similar to tumor cells. Thus, our aforementioned finding can be expected, since this study activated PBMCs with LPS and used 80 µM of BrPA. Furthermore, given that cytotoxic immunosuppressive drugs are currently used to treat BD1, BrPA's cytotoxicity may be acceptable for developing it as a supplementary treatment for BD.
We demonstrated that butyrate treatment inhibited IL-6, TNF-α and IL-1β protein expression in PBMCs of all subjects at 6 hours. Nastasi et al.10 reported that butyrate inhibited the expression of LPS-induced cytokines such as IL-6, IL-12p40, but did not affect LPS-stimulated HLA-DR, CD86, IL-1β expression in human dendritic cells. Compared to our study using 10 mM butyrate, they used 1 mM butyrate and analyzed the effect at 24 hours after treatment10. We do not know if the suppressive effect of butyrate on IL-1β expression is dependent on the butyrate concentration, the time point of analysis, or the cell type. Nevertheless, consistent with our results, a recent report demonstrates butyrate decreases Nlrp3 inflammasome formation and activation in in vivo as well as in vitro endothelial cells through blocking lipid raft signaling platforms11. Further, anti-inflammatory effect of butyrate is manifold such as reducing the release of pro-inflammatory chemokines (CCL3, CCL4, CCL5, CXCL9, CXCL10 and CXCL11)10 and enhancing the release of the anti-inflammatory cytokine IL-1012.
We observed that the FFAR2 mRNA level was significantly lower in ex vivo PBMCs of BD patients compared with HC. This was unexpected as the butyrate response was slightly greater in PBMCs of BD patients. However, LPS stimulation of PBMCs increased FFAR2 mRNA expression to a greater degree in BD patients compared to HC. Further study to analyze FFAR2 and FFAR3 protein expression is required. Also, GPR109a (another SCFA receptor) expression analysis in PBMCs of BD patients will be informative given that butyrate significantly ameliorated the inflammatory response in a GPR109a dependent manner through inhibiting AKT and NFκB p65 signaling in inflammatory bowel disease model mice13.
Given the increase in TH17 cell frequency and decrease in regulatory T cell frequency in BD patients1415, it is noteworthy that BrPA inhibits TH17 cell differentiation and promotes regulatory T cell differentiation4. Also, butyrate promotes peripheral regulatory T cell differentiation1617. We analyzed the effect of bromopyruvate on regulatory T cell differentiation in a preliminary study and found that the enhancing effect of bromopyruvate on the Treg-cell differentiation depended on the stimulatory strength and the analysis time point. Further, bromopyruvate concentration and duration to increase Treg-cell differentiation are different from the concentration and duration to suppress inflammatory cytokine production. It was therefore difficult to analyze all effects of bromopyruvate and butyrate on Treg-cell and TH17 cell differentiation, and on inflammatory cytokine production using the limited blood cell numbers obtained from BD patients.
Our study however, was conducted in a small sample without in vivo analysis. Therefore, future research should assess the in vivo effect of bromopyruvate and butyrate in a BD mouse model to clarify 1) whether the bromopyruvate-mediated cell death induction can be tolerated and 2) whether the butyrate-medicated SCFA receptor downregulation constrains the therapeutic effect of butyrate. Notably, a report argues that bromopyruvate was successfully tried in a single patient with a fibrolamellar hepatocellular carcinoma through the blood vessels supplying the tumor18. Further, SCFA receptor synthetic agonist suppresses inflammatory cytokine production in human dendritic cells19 and butyrate administration reduces disease activity in an ulcerative colitis mouse model20. Conclusively, along with the aforementioned reports, we propose bromopyruvate and butyrate as supplementary therapeutic candidates to control inflammation in patients with BD.
ACKNOWLEDGMENT
We thank BA Jeewon Oh for English editing. We also thank Office of Biostatistics, Institute of Medical Sciences, Ajou University School of Medicine for the statistical consult.
This research was supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (grant number. 20fol4M3A9B6069341).
SJ Yun performed all experiments and statistical analysis of all data, and produced figures. K Kim critically reviewed the manuscript. ES Lee recruited study subjects, evaluated patient status and provided clinical information of patients. S Park conceived the study, designed the experiments and wrote the manuscript. All authors read and approved the final manuscript.
References
1. Alpsoy E. Behçet's disease: a comprehensive review with a focus on epidemiology, etiology and clinical features, and management of mucocutaneous lesions. J Dermatol. 2016; 43:620–632. PMID: 27075942.

2. Loftus RM, Finlay DK. Immunometabolism: cellular metabolism turns immune regulator. J Biol Chem. 2016; 291:1–10. PMID: 26534957.

3. Seki SM, Stevenson M, Rosen AM, Arandjelovic S, Gemta L, Bullock TNJ, et al. Lineage-specific metabolic properties and vulnerabilities of T cells in the demyelinating central nervous system. J Immunol. 2017; 198:4607–4617. PMID: 28507026.

4. Okano T, Saegusa J, Nishimura K, Takahashi S, Sendo S, Ueda Y, et al. 3-bromopyruvate ameliorate autoimmune arthritis by modulating Th17/Treg cell differentiation and suppressing dendritic cell activation. Sci Rep. 2017; 7:42412. PMID: 28186160.

5. Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016; 57:943–954. PMID: 27080715.

6. Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016; 5:e73. PMID: 27195116.

7. Consolandi C, Turroni S, Emmi G, Severgnini M, Fiori J, Peano C, et al. Behçet's syndrome patients exhibit specific microbiome signature. Autoimmun Rev. 2015; 14:269–276. PMID: 25435420.

8. Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, et al. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004; 324:269–275. PMID: 15465013.

9. Lachmandas E, Boutens L, Ratter JM, Hijmans A, Hooiveld GJ, Joosten LA, et al. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat Microbiol. 2016; 2:16246. PMID: 27991883.

10. Nastasi C, Candela M, Bonefeld CM, Geisler C, Hansen M, Krejsgaard T, et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 2015; 5:16148. PMID: 26541096.

11. Yuan X, Wang L, Bhat OM, Lohner H, Li PL. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: antioxidant action of butyrate. Redox Biol. 2018; 16:21–31. PMID: 29475132.

12. Säemann MD, Parolini O, Böhmig GA, Kelemen P, Krieger PM, Neumüller J, et al. Bacterial metabolite interference with maturation of human monocyte-derived dendritic cells. J Leukoc Biol. 2002; 71:238–246. PMID: 11818444.
13. Chen G, Ran X, Li B, Li Y, He D, Huang B, et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine. 2018; 30:317–325. PMID: 29627390.

14. Geri G, Terrier B, Rosenzwajg M, Wechsler B, Touzot M, Seilhean D, et al. Critical role of IL-21 in modulating TH17 and regulatory T cells in Behçet disease. J Allergy Clin Immunol. 2011; 128:655–664. PMID: 21724243.
15. Na SY, Park MJ, Park S, Lee ES. Up-regulation of Th17 and related cytokines in Behçet's disease corresponding to disease activity. Clin Exp Rheumatol. 2013; 31(3 Suppl 77):32–40. PMID: 24064012.
16. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013; 504:451–455. PMID: 24226773.

17. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013; 504:446–450. PMID: 24226770.

18. Ko YH, Verhoeven HA, Lee MJ, Corbin DJ, Vogl TJ, Pedersen PL. A translational study “case report” on the small molecule “energy blocker” 3-bromopyruvate (3BP) as a potent anticancer agent: from bench side to bedside. J Bioenerg Biomembr. 2012; 44:163–170. PMID: 22328020.

19. Ang Z, Er JZ, Tan NS, Lu J, Liou YC, Grosse J, et al. Human and mouse monocytes display distinct signalling and cytokine profiles upon stimulation with FFAR2/FFAR3 short-chain fatty acid receptor agonists. Sci Rep. 2016; 6:34145. PMID: 27667443.

20. Ji J, Shu D, Zheng M, Wang J, Luo C, Wang Y, et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci Rep. 2016; 6:24838. PMID: 27094081.

Fig. 1
The effect of bromopyruvate and butyrate on the cytokine mRNA expression and the survival of THP-1 cells. Cytokine mRNA levels in lipopolysaccharide (LPS)-stimulated THP-1 cells in the presence or absence of bromopyruvate (BrPA) or butyrate (Buty) were analyzed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Relative mRNA level to the mRNA level at the beginning of culture (A). The percent mRNA level against the mRNA amount in the absence of a drug (B~E). Cell survival (F). Data are presented as median with range of 6 independent experiments with duplication or triplication. IL: interleukin, mRNA: messenger ribonucleic acid, TNF: tumor necrosis factor. *p<0.05, **p<0.01, ***p<0.001.
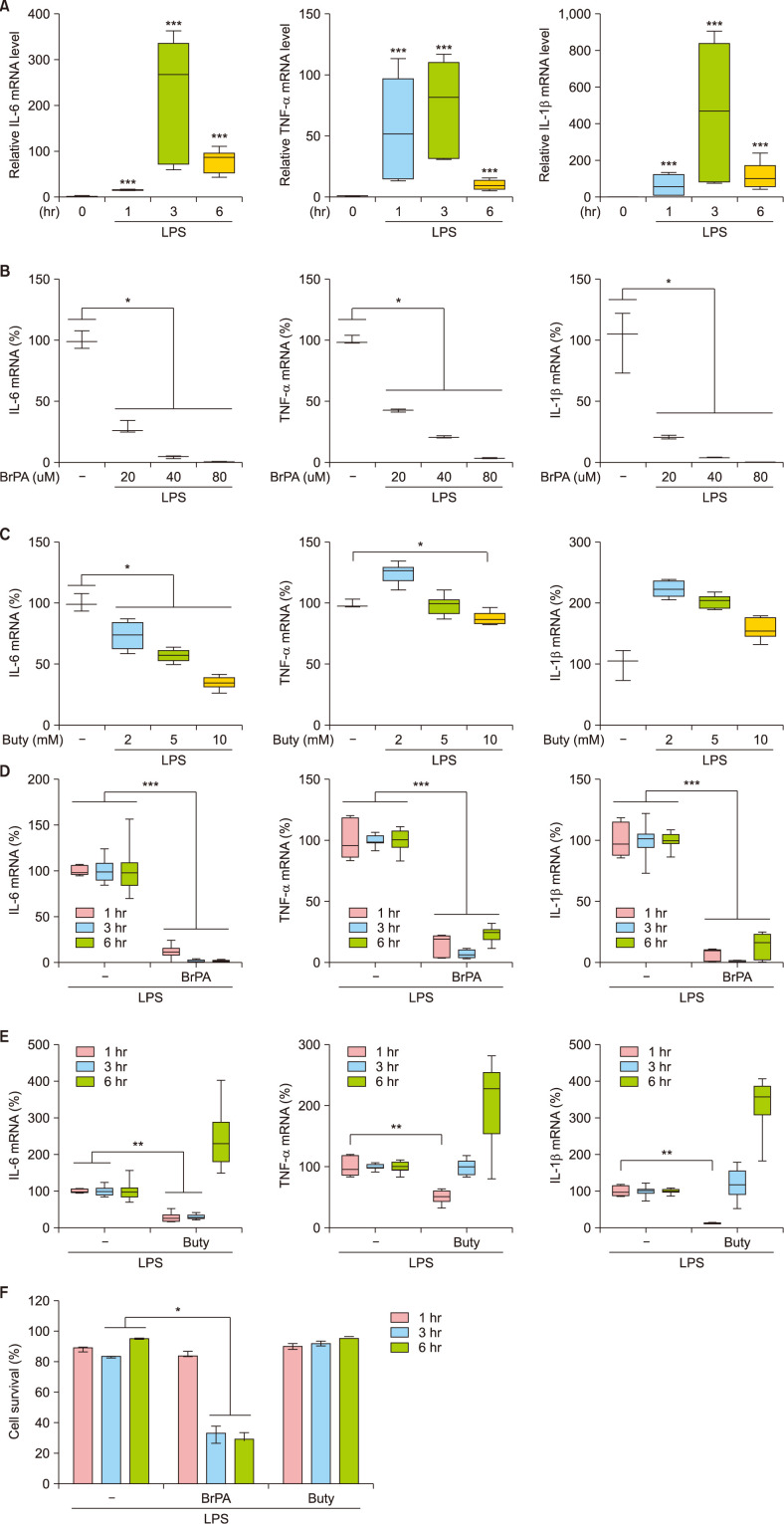
Fig. 2
The effect of bromopyruvate and butyrate on the cytokine expression and the survival of peripheral blood mononuclear cells (PBMCs). PBMCs of patients with Behçet's disease (BD) and healthy controls (HC) were stimulated with lipopolysaccharide (LPS) in the presence or absence of bromopyruvate (BrPA) or butyrate (Buty). The indicated cytokine mRNA (A, C, E) and protein levels (B, D, F) were determined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and Enzyme-linked immunosorbent assay, respectively. The relative mRNA levels in the LPS-stimulated PBMCs against the average mRNA level in unstimulated HC PBMCs (A). The percent mRNA level in the LPS/BrPA or Buty - treated PBMCs against the mRNA level in the LPS-stimulated PBMCs (C, E). The percent protein level in the LPS/BrPA or Buty - treated PBMCs against the protein level in the LPS-stimulated PBMCs (D, F). Cell survival was analyzed by flow cytometry (G). Data are presented as median with range. Data from more than 5 independent experiments with duplication or triplication. IL: interleukin, mRNA: messenger ribonucleic acid, TNF: tumor necrosis factor, Inac. BD: patients with inactive BD, Ac. BD: patients with active BD. *p<0.05, **p<0.01, ***p<0.001.
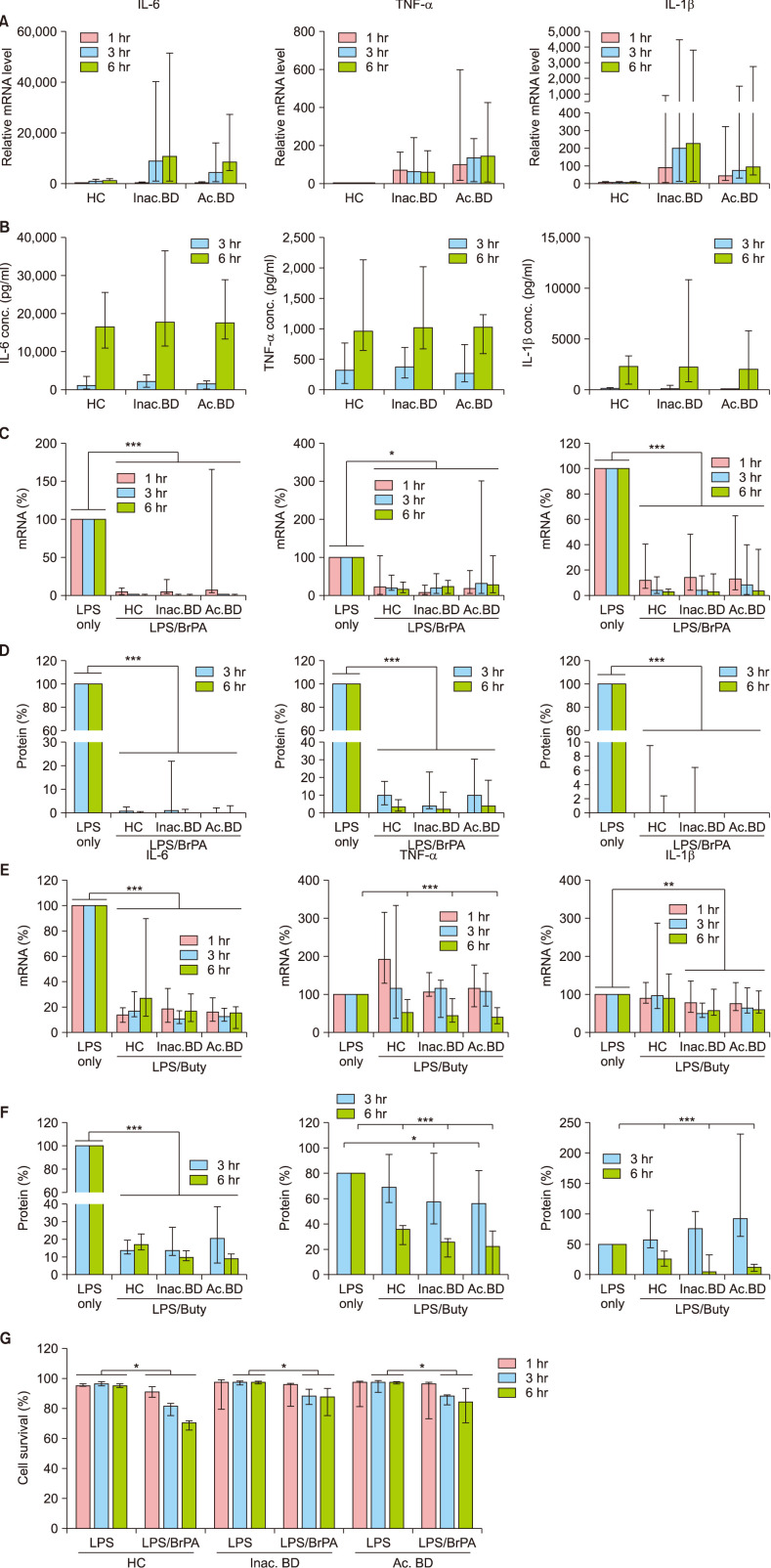
Fig. 3
Free fatty acid receptor 2 (FFAR2) and FFAR3 mRNA expression in peripheral blood mononuclear cells (PBMCs). The relative mRNA level in ex-vivo PBMCs of each subject against the average mRNA amount of healthy controls (HC) (A). The relative mRNA levels in the lipopolysaccharide (LPS)-stimulated PBMCs against the average mRNA level in unstimulated HC PBMCs (B). The percent mRNA level in the LPS/bromopyruvate (BrPA) or butyrate - treated PBMCs against the mRNA level in the LPS-stimulated PBMCs (C, D). Data are presented as median with range of more than 5 independent experiments with duplication or triplication. mRNA: messenger ribonucleic acid, Inac. BD: patients with inactive BD, Ac. BD: patients with active BD. *p<0.05, **p<0.01, ***p<0.001.
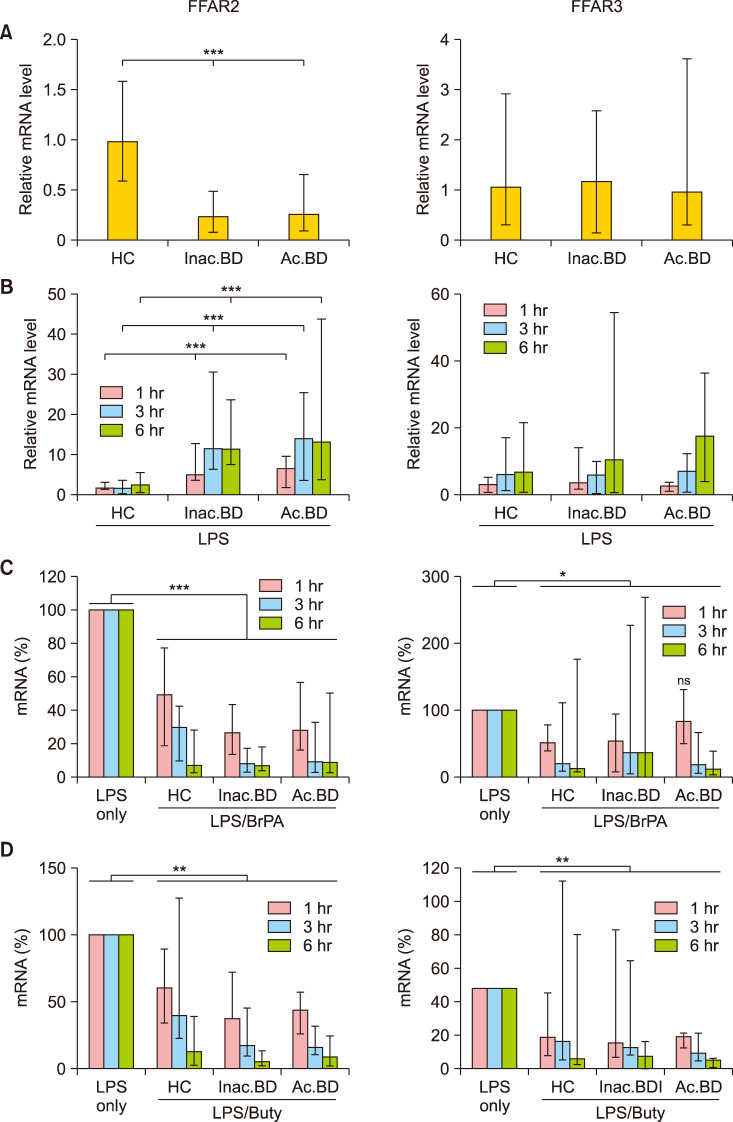
Table 1
The clinical features and medication of BD patients at the time of recruitment





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download