Abstract
Background
The causative agents of leprosy are the well-known Mycobacterium leprae and the newly discovered Mycobacterium lepromatosis. This agent was found in 2008, and it was found to be the cause of diffuse lepromatous leprosy in two Mexican patients.
Objective
The objective of this work was to determine if M. leprae and M. lepromatosis were present in formalin-fixed and paraffin-embedded skin samples from cases from different regions in Mexico.
Methods
A total of 41 skin samples were obtained from 11 states of Mexico. All patients' samples were diagnosed by clinical and histopathological analyses. Total DNA was isolated using a Qiagen-DNeasy blood and tissue kit and molecular identification was achieved by two semi-nested polymerase chain reactions.
Results
The 41 patient included 33 samples from men and 8 samples from women; 29 samples were polymerase chain reaction (PCR)-positive to Mycobacterium and 12 samples were PCR-negative. From those 29 samples, 13 were PCR-positive to M. leprae, 8 to M. lepromatosis and 8 were positive to both species. The histopathological diagnosis included; Nodular lepromatous leprosy (NLL); Diffuse lepromatous leprosy (DLL); and Borderline leprosy (BL). The 29 PCR-positive samples were classified as follow: 14 NLL, 4 DLL, and 11 BL. In the 12 samples negative to Mycobacterium, 7 showed the NLL, 2 DLL and 3 BL.
Leprosy is a mycobacterial infection, which affects primarily the skin, peripheral nerves, eyes, and mucous membranes of the upper respiratory tract1. This disease has plagued humans for millennia and remains a significant public health problem2. Worldwide, it is an outstanding cause of morbidity due to physical handicaps and social stigma. Until 2013, the World Health Organization reported a cases-rate of >10 per 100,000 population in India and Brazil; 1~10 per 100,000 in Africa and Far East; <1 per 100,000 in Latin America, United States, China, Middle East and Australia3. In Mexico, leprosy is an endemic disease in 28 states, with 166 new cases reported at the end of 20164.
The causative agents of leprosy are the well-known Mycobacterium leprae and the newly discovered Mycobacterium lepromatosis2. This new agent was discovered in 2008, and it was found to be the cause of diffuse lepromatous leprosy (DLL) in two Mexican patients. The Mycobacterium bacilli differs at least 9.1% and diverged ~10 million years ago from their last common ancestor5. The M. lepromatosis genome matched ~87% overall with the M. leprae genome (3,268,071 bp)678.
The causative agent is transmitted via airborne droplets or by prolonged skin direct contact with a multibacillary leprosy patient. This disease manifests a wide spectrum of clinic and pathological forms (depending of immune host response) ranging from tuberculoid leprosy (TT), passing the borderline leprosy (BL) forms, to lepromatous leprosy (LL), and an initial stage (indeterminated leprosy). A remarkable geographic variation of clinical aspects also exists; in India and Africa, 90% are TT, in Southeast Asia, the two forms are equally distributed3, whereas in Mexico, over 60% of cases are LL. The objective of this work was to determine if M. leprae and M. lepromatosis were present in formalin-fixed and paraffin-embedded tissue samples from cases from different regions in Mexico.
A total of 41 formalin-fixed and paraffin-embedded skin biopsy samples were obtained from eleven states of Mexico; 16 samples from Yucatan, 8 from Guerrero, 6 from Michoacán, 3 from Guanajuato, 2 from Morelos and one sample from Campeche, Ciudad de Mexico, Estado de Mexico, Oaxaca, Puebla and Quintana Roo. The samples were collected at the Hospital General “Dr. Manuel Gea Gonzalez” and Centro Dermatológico de Yucatan “Dr. Fernando Latapi” from 1994 to 2014. All patients' samples were diagnosed by clinical and histopathological analyses. The clinical and pathological information included the patient's age, sex, localization, biopsy site and date, and histopathological diagnostic. This study was approved by the Institutional Review Board of Hospital General Dr. Manuel Gea González, Mexico City, Mexico (IRB no. 06-54-2015). For DNA extraction, eight to ten sections of five micron thickness of tissue were used from each sample.
The formalin-fixed and paraffin-embedded tissue samples were process to remove paraffin using xylene protocol. Total DNA was isolated using a DNeasy blood and tissue kit (Qiagen, Ventura, CA, USA) according to the manufacturer's instructions. DNA concentration was determined by spectrophotometry at 260 nm. Molecular identification was achieved by two semi-nested PCR, the first PCR used primers AFBFO (5-gcgtgcttaacacatgcaagtc-3) and MLER4 (5-ccacaagacatgcgccttgaag-3). The amplification fragment (171 bp) was used for two separate second-round PCRs using MLER4 and LPMF2 (5-gtctcttaatacttaaacctattaa-3) for M. lepromatosis (142-bp) and MLER4 and LERF2 (5-ctaaaaaatcttttttagagatac-3) for M. leprae (135-bp)29. The PCR reactions contained 25 μl of Top Taq master mix (Qiagen), 100 ng of DNA (10 μl), and 25 μM of each primer (2 μl) in a total volume of 50 μl. Amplification conditions for the first PCR were: initial denaturation at 95℃ for 5 minutes, followed by 30 cycles of denaturing (94℃, 30 s), annealing (57℃, 30 s) and extension (72℃, 30 s), followed by a final extension at 72℃ for 5 minutes. For the second round of PCR's, we use the same protocol with annealing at 53℃. A sample of 10 μl of product from each PCR was electrophoresed in a 3% agarose gel with 0.5 μg of ethidium bromide/ml and 1X Tris-acetate-EDTA buffer for 1 hour. DNA bands were visualized on a UV transilluminator and documented with The Gel Logic 212 Pro Software (Carestream, Woodbridge, CT, USA).
Forty-one patients were diagnosed with leprosy by clinical and histopathological analysis; twenty-nine samples were PCR-positive to Mycobacterium (70.73%) and twelve samples were PCR-negative (29.27%). From those twenty-nine samples, thirteen were PCR-positive to M. leprae (44.83), eight to M. lepromatosis (27.58%) and eight were positive to both species (27.58%).
The forty-one patients included thirty-three samples from men and eight samples from women. The average age was 52 years, range from 23 to 78 years-old. The 29 PCR-positive samples to Mycobacterium; included 13 M. leprae positive (11 men and 2 women), and 8 M. lepromatosis positive (7 men and a woman). The eight patients positive for both species, six were men and two women. From the twelve negative samples, nine were men and three women. Regarding the geographic region; M. leprae was found in six samples from Yucatan, 3 from Michoacán, and one from Mexico City, Mexico state, Guerrero and Puebla (13 samples). M. lepromatosis was found in two samples from Guerrero, 2 from Michoacán, 2 from Yucatan, 1 from Guanajuato, and 1 from Quintana Roo (8 samples). The dual infection was present in six samples from Yucatan, one from Campeche and one from Oaxaca. The twelve negative samples were; five from Guerrero, two from Morelos, two from Guanajuato, two from Yucatan, and one from Michoacan.
The histopathological diagnosis observed included; Nodular lepromatous leprosy (NLL) (twenty-one samples); DLL (six samples); and Borderline leprosy (BL) (fourteen samples). In the twenty nine PCR-positive samples, fourteen showed the NLL form, four showed the DLL form, and eleven the BL form. In the twelve samples negative to Mycobacterium, seven showed the NLL form, two showed the DLL form and three the BL form.
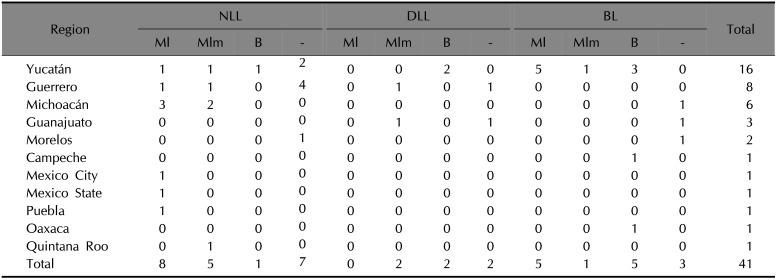
In sum, the twenty-nine positive samples to Mycobacterium showed a distribution profile of eight samples with the NLL form positive to M. leprae, five to M. lepromatosis and one positive to both. The DLL form was present in two positive samples to M. lepromatosis, and two samples positive to both. The BL form was shown in five positive samples to M. leprae, one to M. lepromatosis, and five positive to both (Table 1).
M. leprae was the only known cause of leprosy until 2008, when the long-elusive M. lepromatosis was identified as the second agent in leprosy patients from 12 Mexican states; Tamaulipas, Sonora, Sinaloa, Nayarit, Colima, Michoacan, Guerrero and Queretaro6. Currently, it is the dominant cause of leprosy and considered endemic in the western and central part of Mexico. Globally, the male/female leprosy rate is male dominated 3/210. In Mexico, this rate is 2/1311. In this work, the male dominance was greater up to 4.12/1 (33/8 cases).
Dual infections due to M. lepromatosis and M. leprae also had been reported12, its frequency may be up to 16.1%6. In our study, it was greater, up to 19% (8/41) (Table 1). Interestingly, the origin of most of the dual infections was the Yucatan peninsula, an area known to have frequent leprosy cases11, except for a case from Oaxaca678.
M. lepromatosis has been related as the specific cause of the severe DLL form1314. Since its discovery, its prevalence and significance has raised the scientific interest. According to our findings, M. lepromatosis may cause the NLL and BL (Table 1). Given that M. lepromatosis is not geographically restricted to Mexico as it has been identified in America and Asia; Brazil, Myanmar, Canada, and Singapore121516171819, and that it can participates in dual infections in Leprosy endemic areas, M. lepromatosis should be taken on account for diagnosis worldwide. However as it has been observed in another papers DLL is related only with M. lepromtosis alone or with dual infection, but not only with M. leprae, it could explain the severity of these cases13.
ACKNOWLEDGMENT
We thank Dra. Sonia Toussaint Caire, Jessica Espinoza Hernández and Ixtabay Ilizaliturry for sample histopathological examination assistance.
References
1. Lupi O, Madkan V, Tyring SK. Tropical dermatology: bacterial tropical diseases. J Am Acad Dermatol. 2006; 54:559–578. PMID: 16546577.

2. Han XY, Seo YH, Sizer KC, Schoberle T, May GS, Spencer JS, et al. A new mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008; 130:856–864. PMID: 19019760.
3. World Health Organization. Global leprosy update, 2015: time for action, accountability and inclusion. Wkly Epidemiol Rec. 2016; 91:405–420.
4. Secretaria de la Salud. Dirección General de Epidemiología [Internet]. Mexico City: GOB.MX;2016. updated 2016 Mar 7. cited 2018 Apr 3. Available from: https://www.gob.mx/salud/acciones-y-programas/direccion-general-deepidemiologia.
5. Han XY, Sizer KC, Thompson EJ, Kabanja J, Li J, Hu P, et al. Comparative sequence analysis of mycobacterium leprae and the new leprosy-causing mycobacterium lepromatosis. J Bacteriol. 2009; 191:6067–6074. PMID: 19633074.
6. Han XY, Sizer KC, Velarde-Félix JS, Frias-Castro LO, Vargas-Ocampo F. The leprosy agents mycobacterium lepromatosis and mycobacterium leprae in Mexico. Int J Dermatol. 2012; 51:952–959. PMID: 22788812.

7. Han XY, Mistry NA, Thompson EJ, Tang HL, Khanna K, Zhang L. Draft genome sequence of new leprosy agent mycobacterium lepromatosis. Genome Announc. 2015; 3:e00513–e00515. PMID: 25999555.

8. Singh P, Benjak A, Schuenemann VJ, Herbig A, Avanzi C, Busso P, et al. Insight into the evolution and origin of leprosy bacilli from the genome sequence of mycobacterium lepromatosis. Proc Natl Acad Sci U S A. 2015; 112:4459–4464. PMID: 25831531.

9. Cox RA, Kempsell K, Fairclough L, Colston MJ. The 16S ribosomal RNA of mycobacterium leprae contains a unique sequence which can be used for identification by the polymerase chain reaction. J Med Microbiol. 1991; 35:284–290. PMID: 1719203.
10. Pfaltzgraff RE. What is the actual male/female sex ratio in leprosy patients? Lepr Rev. 2003; 74:180–181. PMID: 12862262.
11. Atoche C, Torres-Guerrero E, Vargas F, Arenas R. [Leprosy in Yucatan, retrospective clinical study of 63 years (1950–2013)]. Salud Publica Mex. 2015; 57:191–192. Spanish. PMID: 26302115.
12. Han XY, Aung FM, Choon SE, Werner B. Analysis of the leprosy agents mycobacterium leprae and mycobacterium lepromatosis in four countries. Am J Clin Pathol. 2014; 142:524–532. PMID: 25239420.

13. Vera-Cabrera L, Escalante-Fuentes WG, Gomez-Flores M, Ocampo-Candiani J, Busso P, Singh P, et al. Case of diffuse lepromatous leprosy associated with “mycobacterium lepromatosis”. J Clin Microbiol. 2011; 49:4366–4368. PMID: 22012006.

14. Vera-Cabrera L, Escalante-Fuentes W, Ocampo-Garza SS, Ocampo-Candiani J, Molina-Torres CA, Avanzi C, et al. Mycobacterium lepromatosis infections in Nuevo León, Mexico. J Clin Microbiol. 2015; 53:1945–1946. PMID: 25809978.

15. Han XY, Sizer KC, Tan HH. Identification of the leprosy agent mycobacterium lepromatosis in Singapore. J Drugs Dermatol. 2012; 11:168–172. PMID: 22270197.
16. Jessamine PG, Desjardins M, Gillis T, Scollard D, Jamieson F, Broukhanski G, et al. Leprosy-like illness in a patient with mycobacterium lepromatosis from Ontario, Canada. J Drugs Dermatol. 2012; 11:229–233. PMID: 22270208.
17. Han XY. Detection of the leprosy agent mycobacterium lepromatosis in South America and Europe. Am J Trop Med Hyg. 2017; 96:260. PMID: 27879462.
18. Sotiriou MC, Stryjewska BM, Hill C. Two cases of leprosy in siblings caused by mycobacterium lepromatosis and review of the literature. Am J Trop Med Hyg. 2016; 95:522–527. PMID: 27402522.

19. Virk A, Pritt B, Patel R, Uhl JR, Bezalel SA, Gibson LE, et al. Mycobacterium lepromatosis lepromatous leprosy in US citizen who traveled to disease-endemic areas. Emerg Infect Dis. 2017; 23:1864–1866. PMID: 29048278.
20. Gillis TP, Scollard DM, Lockwood DN. What is the evidence that the putative mycobacterium lepromatosis species causes diffuse lepromatous leprosy? Lepr Rev. 2011; 82:205–209. PMID: 22125927.

21. Herath S, Navinan MR, Liyanage I, Rathnayaka N, Yudhishdran J, Fernando J, et al. Lucio's phenomenon, an uncommon occurrence among leprosy patients in Sri Lanka. BMC Res Notes. 2015; 8:672. PMID: 26566619.

Table 1
Histopathological findings and geographical distribution




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download