Abstract
Background
Methods
Results
References
Table 1
Performance of TST and various IGRAs in HIV infected individuals with evidence of active TB
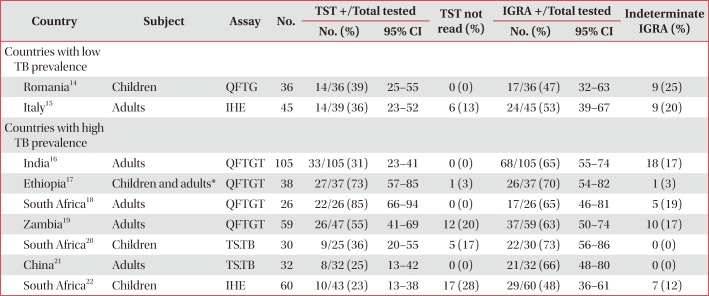
| Country | Subject | Assay | No. | TST +/Total tested | TST not read (%) | IGRA +/Total tested | Indeterminate IGRA (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | 95% CI | No. (%) | 95% CI | ||||||
| Countries with low TB prevalence | |||||||||
| Romania14 | Children | QFTG | 36 | 14/36 (39) | 25–55 | 0 (0) | 17/36 (47) | 32–63 | 9 (25) |
| Italy15 | Adults | IHE | 45 | 14/39 (36) | 23–52 | 6 (13) | 24/45 (53) | 39–67 | 9 (20) |
| Countries with high TB prevalence | |||||||||
| India16 | Adults | QFTGT | 105 | 33/105 (31) | 23–41 | 0 (0) | 68/105 (65) | 55–74 | 18 (17) |
| Ethiopia17 | Children and adults* | QFTGT | 38 | 27/37 (73) | 57–85 | 1 (3) | 26/37 (70) | 54–82 | 1 (3) |
| South Africa18 | Adults | QFTGT | 26 | 22/26 (85) | 66–94 | 0 (0) | 17/26 (65) | 46–81 | 5 (19) |
| Zambia19 | Adults | QFTGT | 59 | 26/47 (55) | 41–69 | 12 (20) | 37/59 (63) | 50–74 | 10 (17) |
| South Africa20 | Children | TS.TB | 30 | 9/25 (36) | 20–55 | 5 (17) | 22/30 (73) | 56–86 | 0 (0) |
| China21 | Adults | TS.TB | 32 | 8/32 (25) | 13–42 | 0 (0) | 21/32 (66) | 48–80 | 0 (0) |
| South Africa22 | Children | IHE | 60 | 10/43 (23) | 13–38 | 17 (28) | 29/60 (48) | 36–61 | 7 (12) |
Detailed description of methodological differences between studies in Table 2.
*Study population aged 15–60 years old.
TST: tuberculin skin test; IGRA: interferon-γ release assay; HIV: human immunodeficiency virus; TB: tuberculosis; CI: confidence interval; QFTG: QuantiFERON Gold; IHE: in house ELISPOT assay; QFTGT: QuantiFERON Gold in Tube; TS.TB: T-SPOT.TB.
Table 2
Methodological differences between studies comparing TST and various IGRAs in subjects with evidence of active TB disease
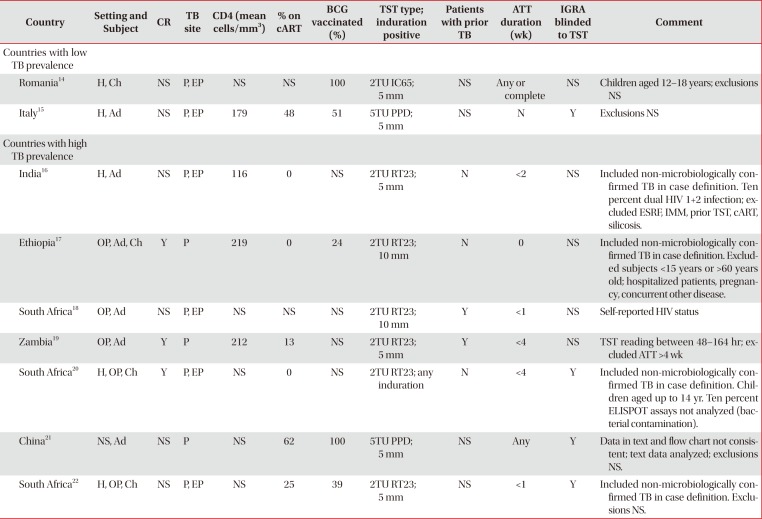
| Country | Setting and Subject | CR | TB site | CD4 (mean cells/mm3) | % on cART | BCG vaccinated (%) | TST type; induration positive | Patients with prior TB | ATT duration (wk) | IGRA blinded to TST | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Countries with low TB prevalence | |||||||||||
| Romania14 | H, Ch | NS | P, EP | NS | NS | 100 | 2TU IC65; 5 mm | NS | Any or complete | NS | Children aged 12–18 years; exclusions NS |
| Italy15 | H, Ad | NS | P, EP | 179 | 48 | 51 | 5TU PPD; 5 mm | NS | N | Y | Exclusions NS |
| Countries with high TB prevalence | |||||||||||
| India16 | H, Ad | NS | P, EP | 116 | 0 | NS | 2TU RT23; 5 mm | N | <2 | NS | Included non-microbiologically confirmed TB in case definition. Ten percent dual HIV 1+2 infection; excluded ESRF, IMM, prior TST, cART, silicosis. |
| Ethiopia17 | OP, Ad, Ch | Y | P | 219 | 0 | 24 | 2TU RT23; 10 mm | N | 0 | NS | Included non-microbiologically confirmed TB in case definition. Excluded subjects <15 years or >60 years old; hospitalized patients, pregnancy, concurrent other disease. |
| South Africa18 | OP, Ad | NS | P, EP | NS | NS | NS | 2TU RT23; 10 mm | Y | <1 | NS | Self-reported HIV status |
| Zambia19 | OP, Ad | Y | P | 212 | 13 | NS | 2TU RT23; 5 mm | Y | <4 | NS | TST reading between 48–164 hr; excluded ATT >4 wk |
| South Africa20 | H, OP, Ch | Y | P, EP | NS | 0 | NS | 2TU RT23; any induration | N | <4 | Y | Included non-microbiologically confirmed TB in case definition. Children aged up to 14 yr. Ten percent ELISPOT assays not analyzed (bacterial contamination). |
| China21 | NS, Ad | NS | P | NS | 62 | 100 | 5TU PPD; 5 mm | NS | Any | Y | Data in text and flow chart not consistent; text data analyzed; exclusions NS. |
| South Africa22 | H, OP, Ch | NS | P, EP | NS | 25 | 39 | 2TU RT23; 5 mm | NS | <1 | Y | Included non-microbiologically confirmed TB in case definition. Exclusions NS. |
TST: tuberculin skin test; IGRA: interferon-γ release assay; TB: tuberculosis; CR: consecutive recruitment; cART: combination antiretroviral therapy; BCG: bacille Calmette-Guerin; ATT: anti-tuberculous therapy; H: hospital; Ch: children; NS: not stated in text; P: pulmonary TB; EP: extra pulmonary TB; TU IC65: Romanian purified protein derivative; Ad: adults; N: no; Y: yes; PPD: purified protein derivative; TU RT23: international tuberculin units of purified protein derivative Staten Serum Institute, Copenhagen, Denmark; HIV: human immunodeficiency virus; ESRF: end stage renal failure; IMM: immunosuppressant including steroids; OP: outpatient.
Table 3
Performance of TST and various IGRAs in HIV infected individuals without evidence of active TB infection
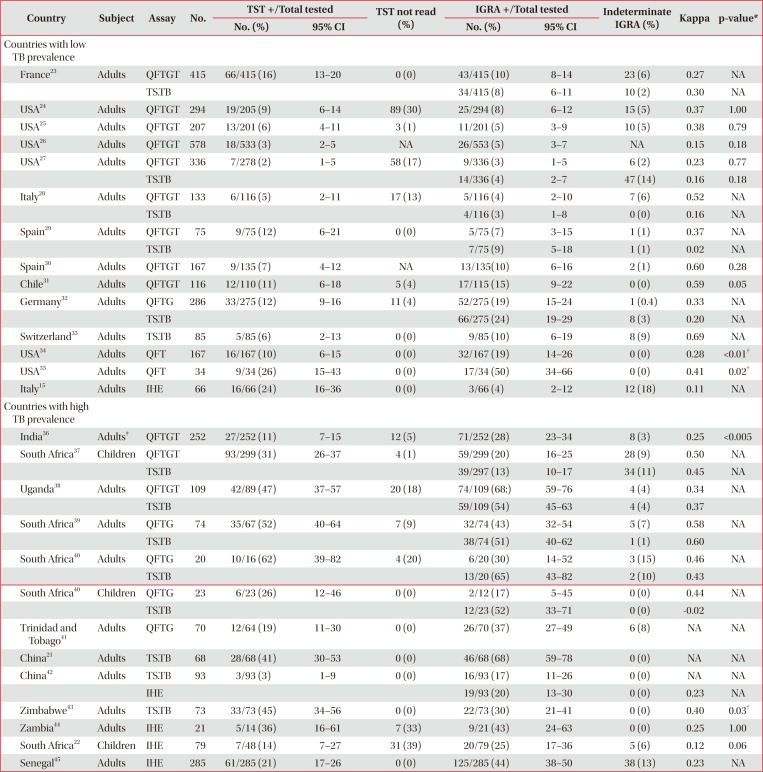
| Country | Subject | Assay | No. | TST +/Total tested | TST not read (%) | IGRA +/Total tested | Indeterminate IGRA (%) | Kappa | p-value* | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | 95% CI | No. (%) | 95% CI | ||||||||
| Countries with low TB prevalence | |||||||||||
| France23 | Adults | QFTGT | 415 | 66/415 (16) | 13–20 | 0 (0) | 43/415 (10) | 8–14 | 23 (6) | 0.27 | NA |
| TS.TB | 34/415 (8) | 6–11 | 10 (2) | 0.30 | NA | ||||||
| USA24 | Adults | QFTGT | 294 | 19/205 (9) | 6–14 | 89 (30) | 25/294 (8) | 6–12 | 15 (5) | 0.37 | 1.00 |
| USA25 | Adults | QFTGT | 207 | 13/201 (6) | 4–11 | 3 (1) | 11/201 (5) | 3–9 | 10 (5) | 0.38 | 0.79 |
| USA26 | Adults | QFTGT | 578 | 18/533 (3) | 2–5 | NA | 26/553 (5) | 3–7 | NA | 0.15 | 0.18 |
| USA27 | Adults | QFTGT | 336 | 7/278 (2) | 1–5 | 58 (17) | 9/336 (3) | 1–5 | 6 (2) | 0.23 | 0.77 |
| TS.TB | 14/336 (4) | 2–7 | 47 (14) | 0.16 | 0.18 | ||||||
| Italy28 | Adults | QFTGT | 133 | 6/116 (5) | 2–11 | 17 (13) | 5/116 (4) | 2–10 | 7 (6) | 0.52 | NA |
| TS.TB | 4/116 (3) | 1–8 | 0 (0) | 0.16 | NA | ||||||
| Spain29 | Adults | QFTGT | 75 | 9/75 (12) | 6–21 | 0 (0) | 5/75 (7) | 3–15 | 1 (1) | 0.37 | NA |
| TS.TB | 7/75 (9) | 5–18 | 1 (1) | 0.02 | NA | ||||||
| Spain30 | Adults | QFTGT | 167 | 9/135 (7) | 4–12 | NA | 13/135(10) | 6–16 | 2 (1) | 0.60 | 0.28 |
| Chile31 | Adults | QFTGT | 116 | 12/110 (11) | 6–18 | 5 (4) | 17/115 (15) | 9–22 | 0 (0) | 0.59 | 0.05 |
| Germany32 | Adults | QFTG | 286 | 33/275 (12) | 9–16 | 11 (4) | 52/275 (19) | 15–24 | 1 (0.4) | 0.33 | NA |
| TS.TB | 66/275 (24) | 19–29 | 8 (3) | 0.20 | NA | ||||||
| Switzerland33 | Adults | TS.TB | 85 | 5/85 (6) | 2–13 | 0 (0) | 9/85 (10) | 6–19 | 8 (9) | 0.69 | NA |
| USA34 | Adults | QFT | 167 | 16/167 (10) | 6–15 | 0 (0) | 32/167 (19) | 14–26 | 0 (0) | 0.28 | <0.01† |
| USA35 | Adults | QFT | 34 | 9/34 (26) | 15–43 | 0 (0) | 17/34 (50) | 34–66 | 0 (0) | 0.41 | 0.02† |
| Italy15 | Adults | IHE | 66 | 16/66 (24) | 16–36 | 0 (0) | 3/66 (4) | 2–12 | 12 (18) | 0.11 | NA |
| Countries with high TB prevalence | |||||||||||
| India36 | Adults‡ | QFTGT | 252 | 27/252 (11) | 7–15 | 12 (5) | 71/252 (28) | 23–34 | 8 (3) | 0.25 | <0.005 |
| South Africa37 | Children | QFTGT | 93/299 (31) | 26–37 | 4 (1) | 59/299 (20) | 16–25 | 28 (9) | 0.50 | NA | |
| TS.TB | 39/297 (13) | 10–17 | 34 (11) | 0.45 | NA | ||||||
| Uganda38 | Adults | QFTGT | 109 | 42/89 (47) | 37–57 | 20 (18) | 74/109 (68;) | 59–76 | 4 (4) | 0.34 | NA |
| TS.TB | 59/109 (54) | 45–63 | 4 (4) | 0.37 | |||||||
| South Africa39 | Adults | QFTG | 74 | 35/67 (52) | 40–64 | 7 (9) | 32/74 (43) | 32–54 | 5 (7) | 0.58 | NA |
| TS.TB | 38/74 (51) | 40–62 | 1 (1) | 0.60 | |||||||
| South Africa40 | Adults | QFTG | 20 | 10/16 (62) | 39–82 | 4 (20) | 6/20 (30) | 14–52 | 3 (15) | 0.46 | NA |
| TS.TB | 13/20 (65) | 43–82 | 2 (10) | 0.43 | |||||||
| South Africa40 | Children | QFTG | 23 | 6/23 (26) | 12–46 | 0 (0) | 2/12 (17) | 5–45 | 0 (0) | 0.44 | NA |
| TS.TB | 12/23 (52) | 33–71 | 0 (0) | −0.02 | |||||||
| Trinidad and Tobago41 | Adults | QFTG | 70 | 12/64 (19) | 11–30 | 0 (0) | 26/70 (37) | 27–49 | 6 (8) | NA | NA |
| China21 | Adults | TS.TB | 68 | 28/68 (41) | 30–53 | 0 (0) | 46/68 (68) | 59–78 | 0 (0) | NA | NA |
| China42 | Adults | TS.TB | 93 | 3/93 (3) | 1–9 | 0 (0) | 16/93 (17) | 11–26 | 0 (0) | NA | NA |
| IHE | 19/93 (20) | 13–30 | 0 (0) | 0.23 | NA | ||||||
| Zimbabwe43 | Adults | TS.TB | 73 | 33/73 (45) | 34–56 | 0 (0) | 22/73 (30) | 21–41 | 0 (0) | 0.40 | 0.03† |
| Zambia44 | Adults | IHE | 21 | 5/14 (36) | 16–61 | 7 (33) | 9/21 (43) | 24–63 | 0 (0) | 0.25 | 1.00 |
| South Africa22 | Children | IHE | 79 | 7/48 (14) | 7–27 | 31 (39) | 20/79 (25) | 17–36 | 5 (6) | 0.12 | 0.06 |
| Senegal45 | Adults | IHE | 285 | 61/285 (21) | 17–26 | 0 (0) | 125/285 (44) | 38–50 | 38 (13) | 0.23 | NA |
Detailed description of methodological differences between studies in Table 4.
*McNemars test. †Statistically significant. ‡Pregnant women.
TST: tuberculin skin test; IGRA: interferon-γ release assay; HIV: human immunodeficiency virus; TB: tuberculosis; QFTGT: QuantiFERON Gold in Tube; TS.TB: T-SPOT.TB; NA: not available; QFTG: QuantiFERON Gold; QFT: QuantiFERON; IHE: in house ELISPOT assay.
Table 4
Methodological and population differences between studies comparing TST and various IGRAs in subjects without evidence of active TB infection
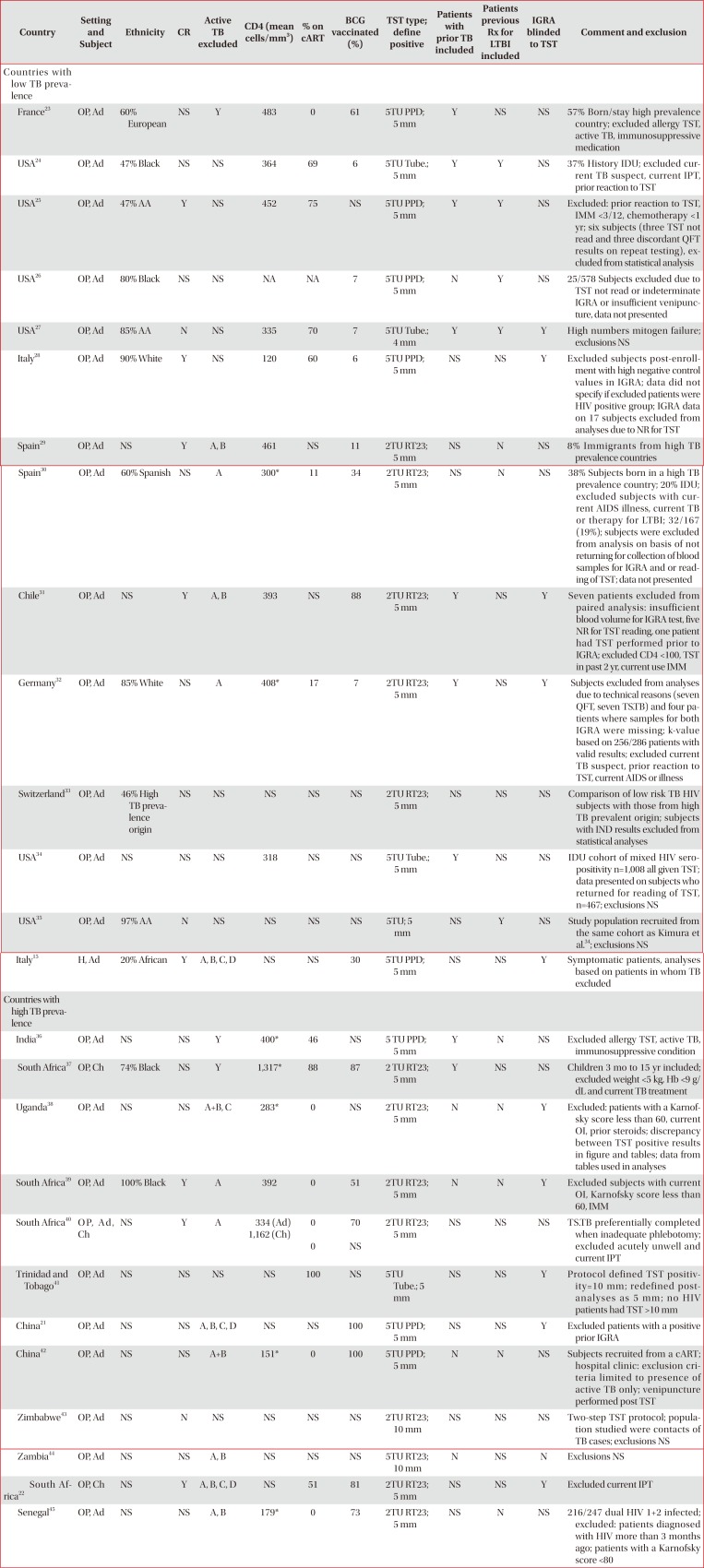
| Country | Setting and Subject | Ethnicity | CR | Active TB excluded | CD4 (mean cells/mm3) | % on cART | BCG vaccinated (%) | TST type; define positive | Patients with prior TB included | Patients previous Rx for LTBI included | IGRA blinded to TST | Comment and exclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Countries with low TB prevalence | ||||||||||||
| France23 | OP, Ad | 60% European | NS | Y | 483 | 0 | 61 | 5TU PPD; 5 mm | Y | NS | NS | 57% Born/stay high prevalence country; excluded allergy TST, active TB, immunosuppressive medication |
| USA24 | OP, Ad | 47% Black | NS | NS | 364 | 69 | 6 | 5TU Tube.; 5 mm | Y | Y | NS | 37% History IDU; excluded current TB suspect, current IPT, prior reaction to TST |
| USA25 | OP, Ad | 47% AA | Y | NS | 452 | 75 | NS | 5TU PPD; 5 mm | Y | Y | NS | Excluded: prior reaction to TST, IMM <3/12, chemotherapy <1 yr; six subjects (three TST not read and three discordant QFT results on repeat testing), excluded from statistical analysis |
| USA26 | OP, Ad | 80% Black | NS | NS | NA | NA | 7 | 5TU PPD; 5 mm | N | Y | NS | 25/578 Subjects excluded due to TST not read or indeterminate IGRA or insufficient venipuncture, data not presented |
| USA27 | OP, Ad | 85% AA | N | NS | 335 | 70 | 7 | 5TU Tube.; 4 mm | Y | Y | Y | High numbers mitogen failure; exclusions NS |
| Italy28 | OP, Ad | 90% White | Y | NS | 120 | 60 | 6 | 5TU PPD; 5 mm | NS | NS | Y | Excluded subjects post-enrollment with high negative control values in IGRA; data did not specify if excluded patients were HIV positive group; IGRA data on 17 subjects excluded from analyses due to NR for TST |
| Spain29 | OP, Ad | NS | Y | A, B | 461 | NS | 11 | 2TU RT23; 5 mm | NS | N | NS | 8% Immigrants from high TB prevalence countries |
| Spain30 | OP, Ad | 60% Spanish | NS | A | 300* | 11 | 34 | 2TU RT23; 5 mm | NS | N | NS | 38% Subjects born in a high TB prevalence country; 20% IDU; excluded subjects with current AIDS illness, current TB or therapy for LTBI; 32/167 (19%); subjects were excluded from analysis on basis of not returning for collection of blood samples for IGRA and or reading of TST; data not presented |
| Chile31 | OP, Ad | NS | Y | A, B | 393 | NS | 88 | 2TU RT23; 5 mm | Y | NS | Y | Seven patients excluded from paired analysis: insufficient blood volume for IGRA test, five NR for TST reading, one patient had TST performed prior to IGRA; excluded CD4 <100, TST in past 2 yr, current use IMM |
| Germany32 | OP, Ad | 85% White | NS | A | 408* | 17 | 7 | 2TU RT23; 5 mm | Y | NS | Y | Subjects excluded from analyses due to technical reasons (seven QFT, seven TS.TB) and four patients where samples for both IGRA were missing; k-value based on 256/286 patients with valid results; excluded current TB suspect, prior reaction to TST, current AIDS or illness |
| Switzerland33 | OP, Ad | 46% High TB prevalence origin | NS | NS | NS | NS | NS | 2TU RT23; 5 mm | NS | NS | NS | Comparison of low risk TB HIV subjects with those from high TB prevalent origin; subjects with IND results excluded from statistical analyses |
| USA34 | OP, Ad | NS | NS | NS | 318 | NS | NS | 5TU Tube.; 5 mm | Y | NS | NS | IDU cohort of mixed HIV seropositivity n=1,008 all given TST; data presented on subjects who returned for reading of TST, n=467; exclusions NS |
| USA35 | OP, Ad | 97% AA | N | NS | NS | NS | NS | 5TU; 5 mm | NS | Y | NS | Study population recruited from the same cohort as Kimura et al.34; exclusions NS |
| Italy15 | H, Ad | 20% African | Y | A, B, C, D | NS | NS | 30 | 5TU PPD; 5 mm | NS | NS | Y | Symptomatic patients, analyses based on patients in whom TB excluded |
| Countries with high TB prevalence | ||||||||||||
| India36 | OP, Ad | NS | NS | Y | 400* | 46 | NS | 5 TU PPD; 5 mm | Y | N | NS | Excluded allergy TST, active TB, immunosuppressive condition |
| South Africa37 | OP, Ch | 74% Black | NS | Y | 1,317* | 88 | 87 | 2 TU RT23; 5 mm | Y | NS | NS | Children 3 mo to 15 yr included; excluded weight <5 kg, Hb <9 g/dL and current TB treatment |
| Uganda38 | OP, Ad | NS | NS | A+B, C | 283* | 0 | NS | 2TU RT23; 5 mm | N | N | Y | Excluded: patients with a Karnofsky score less than 60, current OI, prior steroids; discrepancy between TST positive results in figure and tables; data from tables used in analyses |
| South Africa39 | OP, Ad | 100% Black | Y | A | 392 | 0 | 51 | 2TU RT23; 5 mm | N | N | Y | Excluded subjects with current OI, Karnofsky score less than 60, IMM |
| South Africa40 | O P, A d, Ch | NS | Y | A | 334 (Ad) | 0 | 70 | 2TU RT23; 5 mm | NS | NS | NS | TS.TB preferentially completed when inadequate phlebotomy; excluded acutely unwell and current IPT |
| 1,162 (Ch) | 0 | NS | ||||||||||
| Trinidad and Tobago41 | OP, Ad | NS | NS | NS | NS | 100 | NS | 5TU Tube.; 5 mm | NS | NS | Y | Protocol defined TST positivity=10 mm ; redefined post-analyses as 5 mm ; no HIV patients had TST >10 mm |
| China21 | OP, Ad | NS | NS | A, B, C, D | NS | NS | 100 | 5TU PPD; 5 mm | NS | NS | Y | Excluded patients with a positive prior IGRA |
| China42 | OP, Ad | NS | NS | A+B | 151* | 0 | 100 | 5TU PPD; 5 mm | N | N | NS | Subjects recruited from a cART; hospital clinic: exclusion criteria limited to presence of active TB only; venipuncture performed post TST |
| Zimbabwe43 | OP, Ad | NS | N | NS | NS | NS | NS | 2TU RT23; 10 mm | NS | NS | NS | Two-step TST protocol; population studied were contacts of TB cases; exclusions NS |
| Zambia44 | OP, Ad | NS | NS | A, B | NS | NS | NS | 5TU RT23; 10 mm | N | NS | N | Exclusions NS |
| South Africa22 | OP, Ch | NS | Y | A, B, C, D | NS | 51 | 81 | 2TU RT23; 5 mm | NS | NS | Y | Excluded current IPT |
| Senegal45 | OP, Ad | NS | NS | A, B | 179* | 0 | 73 | 2TU RT23; 5 mm | NS | N | NS | 216/247 dual HIV 1+2 infected; excluded: patients diagnosed with HIV more than 3 months ago; patients with a Karnofsky core <80 |
*Median.
TST: tuberculin skin test; Rx: radiotherapy; IGRA: interferon-γ release assay; TB: tuberculosis; CR: consecutive recruitment; cART: combination antiretroviral therapy; BCG: bacille Calmette-Guerin; LTBI: latent tuberculosis infection; OP: outpatient; Ad: adults; NS: not stated in text; Y: yes; N: no; TU: international tuberculin units; PPD: purified protein derivative; Tube.: tubersol purified protein derivative; IDU: injecting drug use; IPT: isoniazid preventative therapy; AA: African American; IMM: immunosuppressant including steroids; QFT: QuantiFERON-TB; A: clinical evidence of TB; B: radiological features consistent with TB; TU RT23: international tuberculin units of purified protein derivative Staten Serum Institute, Copenhagen, Denmark; AIDS: acquired immune deficiency syndrome; TS.TB: T-SPOT.TB; HIV: human immunodeficiency virus; H: hospital; C: acid fast bacilli-positive smear; D: TB Culture positive; OI: opportunistic infection; Ch: children; Hb: hemoglobin.
Table 5
Performance of TST and IGRAs stratified by CD4 T-cell count in patients without active TB infection
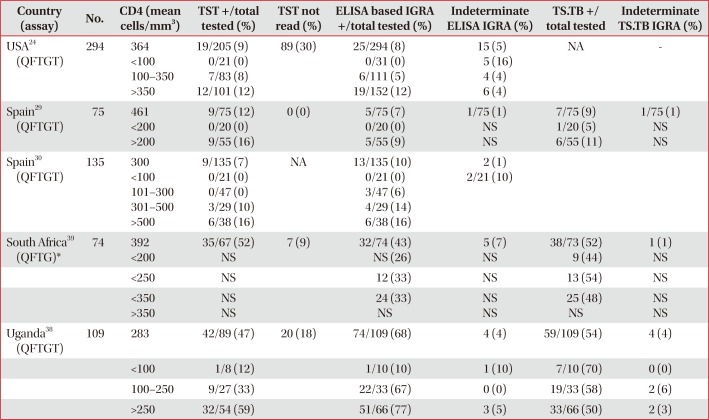
| Country (assay) | No. | CD4 (mean cells/mm3) | TST +/total tested (%) | TST not read (%) | ELISA based IGRA +/total tested (%) | Indeterminate ELISA IGRA (%) | TS.TB +/ total tested | Indeterminate TS.TB IGRA (%) |
|---|---|---|---|---|---|---|---|---|
| USA24 (QFTGT) | 294 | 364 | 19/205 (9) | 89 (30) | 25/294 (8) | 15 (5) | NA | - |
| <100 | 0/21 (0) | 0/31 (0) | 5 (16) | |||||
| 100–350 | 7/83 (8) | 6/111 (5) | 4 (4) | |||||
| >350 | 12/101 (12) | 19/152 (12) | 6 (4) | |||||
| Spain29 (QFTGT) | 75 | 461 | 9/75 (12) | 0 (0) | 5/75 (7) | 1/75 (1) | 7/75 (9) | 1/75 (1) |
| <200 | 0/20 (0) | 0/20 (0) | NS | 1/20 (5) | NS | |||
| >200 | 9/55 (16) | 5/55 (9) | NS | 6/55 (11) | NS | |||
| Spain30 (QFTGT) | 135 | 300 | 9/135 (7) | NA | 13/135 (10) | 2 (1) | ||
| <100 | 0/21 (0) | 0/21 (0) | 2/21 (10) | |||||
| 101–300 | 0/47 (0) | 3/47 (6) | ||||||
| 301–500 | 3/29 (10) | 4/29 (14) | ||||||
| >500 | 6/38 (16) | 6/38 (16) | ||||||
| South Africa39 (QFTG)* | 74 | 392 | 35/67 (52) | 7 (9) | 32/74 (43) | 5 (7) | 38/73 (52) | 1 (1) |
| <200 | NS | NS (26) | NS | 9 (44) | NS | |||
| <250 | NS | 12 (33) | NS | 13 (54) | NS | |||
| <350 | NS | 24 (33) | NS | 25 (48) | NS | |||
| >350 | NS | NS | NS | NS | NS | |||
| Uganda38 (QFTGT) | 109 | 283 | 42/89 (47) | 20 (18) | 74/109 (68) | 4 (4) | 59/109 (54) | 4 (4) |
| <100 | 1/8 (12) | 1/10 (10) | 1 (10) | 7/10 (70) | 0 (0) | |||
| 100–250 | 9/27 (33) | 22/33 (67) | 0 (0) | 19/33 (58) | 2 (6) | |||
| >250 | 32/54 (59) | 51/66 (77) | 3 (5) | 33/66 (50) | 2 (3) |
*Proportion (%) of positive IGRA individuals within CD4 count stratification, denominator not available.
TST: tuberculin skin test; IGRA: interferon-γ release assay; TB: tuberculosis; ELISA: enzyme linked immunosorbent assay; TS.TB: T-SPOT.TB; QFTGT: QuantiFERON Gold in Tube; NA: not available; NS: not stated in text; QFTG: QuantiFERON Gold.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download