Abstract
Objectives
Basal cell carcinoma (BCC) tumors are locally invasive but rarely metastatic. However, aggressive metastatic variants are being increasingly reported in elderly people. Here we investigated the clinical utility of vascular endothelial growth factor (VEGF) as a predictive biomarker for aggressive BCC variants.
Methods
Thirty-five pathologically confirmed cases of BCC that underwent surgical removal in the Plastic Surgery Department between January 1, 2011 and December 31, 2012 were studied. VEGF expression was analyzed in formalin-fixed paraffin-embedded tumor tissue by immunohistochemical staining. Positive staining was defined as more than 10% of the tumor cells showing immunoreactivity. The associations of VEGF expression with various clinicopathologic parameters were analyzed.
Results
The face was the most prevalent site (28/35), with 15 cases from the nose, 6 cases from the eyelid, and 5 cases from the cheek. The patients were aged between 41 and 86 years, with a mean age of 69.26 ± 173.903 years. The mean BCC size was 1.34 ± 3.853 cm, with a range of 0.3 cm to 12.0 cm. The mean tumor invasion depth from the basement epidermal membrane was 0.17 ± 0.035 cm, with a range of 0.03 cm to 1.10 cm. A mean of 5.66 ± 20.938 intraoperative frozen section slides were examined. VEGF was not expressed in 14 of the 35 patients (40.0%), whereas 42.9% of the patients had low expression and 17.1% of the patients had high expression. VEGF expression was significantly associated with age (P = 0.022), size (P = 0.030), site (P = 0.013), tumor invasion depth (P = 0.019), and number of intraoperatively frozen sections (P = 0.003).
REFERENCES
1.Rogers HW., Weinstock MA., Harris AR., Hinckley MR., Feldman SR., Fleischer AB, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010. 146:283–7.

2.Marghoob A., Kopf AW., Bart RS., Sanfilippo L., Silverman MK., Lee P, et al. Risk of another basal cell carcinoma developing after treatment of a basal cell carcinoma. J Am Acad Dermatol. 1993. 28:22–8. 3. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epideriology, clinical features, diagnosis, histopathology, and mangement. Yale J Biol Med 2015;88: 167-79.

4.Linos E., Chren MM., Stijacic Cenzer I., Covinsky KE. Skin Cancer in U.S. Elderly Adults: Does Life Expectancy Play a Role in Treatment Decisions? J Am Geriatr Soc. 2016. 64:1610–5.
6.Mueller MM., Fusenig NE. Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differentiation. 2002. 70:486–97.

7.Gaitanis G., Bassukas I. Intralesional bevacizumab as in-add adjuvant to immunocryosurery for locally advanced basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014. 28:1117–21.
8.Larcher F., Murillas R., Bolontrade M., Conti CJ., Jorcano JL. VEGF/VPF overexpression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development. Oncogene. 1998. 17:303–11.

9.Wilgus TA., Matthies AM., Radek KA., Dovi JV., Burns AL., Shankar R, et al. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am J Pathol. 2005. 167:1257–66.

10.Beck B., Driessens G., Goossens S., Youssef KK., Kuchnio A., Caauwe A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011. 478:399–403.

11.Lichtenberger BM., Tan PK., Niederleithner H., Ferrara N., Petzelbauer P., Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010. 140:268–79.

12.Zhu JW., Wu XJ., Luo D., Lu ZF., Cai SQ., Zheng M. Activation of VEGFr-2 signaling in response to moderate dose of ultraviolet B promotes survival of normal human keratinocytes. Int J Biochem Cell Biol. 2012. 44:246–56.

13.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992. 3:65–7.
14.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005. 438:932–6. 15. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86: 353-64.

16.Viac J., Palacio S., Schmitt D., Claudy A. Expression of vascular endothelial growth factor in normal epidermis, epithelial tumors and cultured keratinocytes. Arch Dermatol Res. 1997. 289:158–63.

17.Bowden J., Brennan PA., Umar T., Cronin A. Expression of vascular endothelial growth factor in basal cell carcinoma and cutaneous squamous cell carcinoma of the head and neck. J Cutan Pathol. 2002. 29:585–9.

18.Weninger W., Uthman A., Pammer J., Pichler A., Ballaun C., Lang IM, et al. Vascular endothelial growth factor production in normal epidermis and in benign and malignant epithelial skin tumors. Lab Invest. 1996. 75:647–57.
19.Larcher F., Robles AI., Duran H., Murillas R., Quintanilla M., Cano A, et al. Up-regulation of vascular endothelial growth factor/vascular permeability factor in mouse skin carcinogenesis correlates with malignant progression state and activated H-ras expression levels. Carcinogenesis. 1996. 56:5391–6.
20.Hirakawa S., Kodama S., Kunstfeld R., Kajiya K., Brown LF., Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005. 201:1089–99.

21.Branson SV., McClintic E., Ozgur O., Esmaeli B., Yeatts RP. Orbitofacial Metastatic Basal Cell Carcinoma: Report of 10 Cases. Ophthal Plast Reconstr Surg. 2017. 33:213–7.

Fig. 2.
The expression of VEGF by immunostaining in the basal cell carcinoma (x100) (A) Negative expression of VEGF in the basal cell carcinoma (B) Low-expression of VEGF in the basal cell carcinoma (C) High-expression of VEGF in the basal cell carcinoma
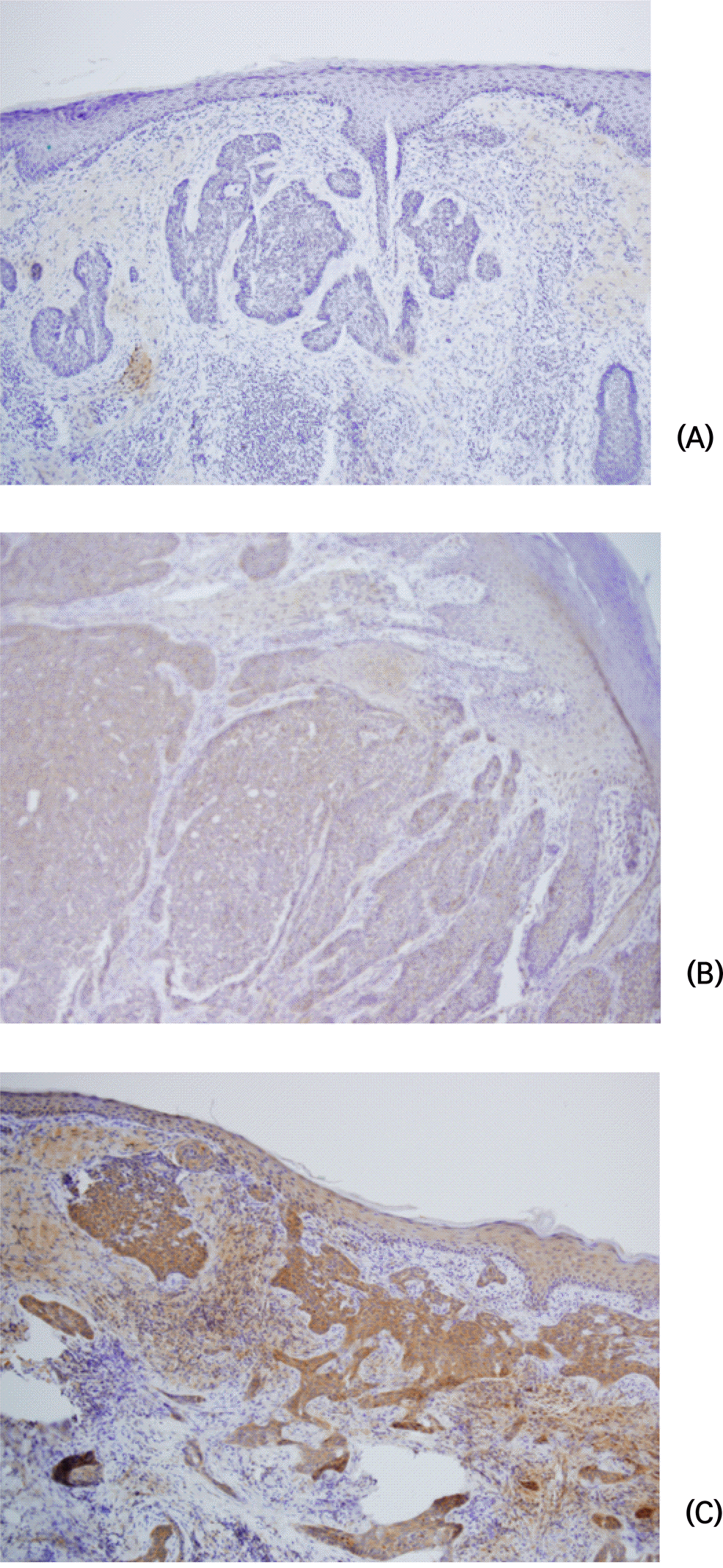
Table 1.
The association of VEGF expression with clinicopathologic variables in the basal cell carinoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download