Abstract
α-Iso-cubebene (ICB) is a dibenzocyclooctadiene lignin contained in Schisandra chinensis (SC), a well-known medicinal herb that ameliorates cardiovascular symptoms, but the mechanism responsible for this activity has not been determined. To determine the role played by ICB on the regulation of vascular tone, we investigated the inhibitory effects of ICB on vascular contractile responses by adrenergic α-receptor agonists. In addition, we investigated the role on myosin light chain (MLC) phosphorylation and cytosolic calcium concentration in vascular smooth muscle cells (VSMC). In aortic rings isolated from C57BL/6J mice, ICB significantly attenuated the contraction induced by phenylephrine (PE) and norepinephrine (NE), whereas ICB had no effects on KCl (60 mM)-induced contraction. In vasculatures precontracted with PE, ICB caused marked relaxation of aortic rings with or without endothelium, suggesting a direct effect on VSMC. In cultured rat VSMC, PE or NE increased MLC phosphorylation and increased cytosolic calcium levels. Both of these effects were significantly suppressed by ICB. In conclusion, our results showed that ICB regulated vascular tone by inhibiting MLC phosphorylation and calcium flux into VSMC, and suggest that ICB has anti-hypertensive properties and therapeutic potential for cardiovascular disorders related to vascular hypertension.
Hypertension is closely associated with the development and presence of cardiovascular diseases, and may result in heart attack, stroke, kidney failure, and disability [1]. Among various risk factors for cardiovascular diseases, vascular tone is an important determinant of peripheral resistance and blood pressure, and essential hypertension is characterized by an abnormal increase in peripheral vascular resistance [23]. Furthermore, prolonged vasoconstriction of a resistant artery is the main driver of vascular remodeling in hypertension [4]. Thus, compounds with resistant artery dilating effects are viewed as being potentially useful for treating hypertension. However, although many different anti-hypertensive drugs are used clinically, new types of medications are required to treat hypertension. Accordingly, much research effort has been directed over recent years to identify novel anti-hypertensive compounds that regulate vascular tone.
It is well known that several mechanisms are involved in the regulation of vascular tone, among which increase in cytoplasmic calcium and myosin light chain (MLC) phorphorylation are important mechanistic features [5]. MLC phosphorylation is determined by the activities of Ca2+-dependent myosin light chain kinase, and is directly linked with smooth muscle contraction [67]. Several authors have shown that MLC phosphatase also contributes to MLC phosphorylation [8910]. Furthermore, in smooth muscle, contractile agonists such as adrenergic alpha-1 agonists increase force generation even when the intracellular concentration of Ca2+ ions is constant, in which MLC phosphatase plays an important role [1112].
Schisandra chinensis has a long history of use as a medicinal herb, and thus, is a component in oriental medicines [1314]. Several researchers have suggested that it may has beneficial effects in patients with cardiovascular diseases, as its aqueous extract induced vasodilation in rat thoracic aorta [1516]. In our previous studies, gomisin A and gomisin J isolated from Schisandra chinensis relaxed vascular smooth muscle, which suggested potential therapeutic use in hypertensive patients [17]. In addition, α-iso-cubebene (ICB), a dibenzocyclooctadiene lignin found in Schisandra chinensis has been suggested as a potential therapeutic intervention to ameliorate the symptoms of cardiovascular disease via its antioxidant property.
Although ICB is known to ameliorate cardiovascular symptoms, but little is known of its effect on the vascular tone of resistant arteries, which is main determinant of vascular hypertension. To determine the effects of ICB on the modulation of vascular tone, the role of ICB on vascular contractile responses by adrenergic alpha-receptor agonists, that is phenylephrine and norepinephrine. Also, the vasodilatory effects of ICB in aortic rings pre-contracted with phenylephrine was determined. In a mechanistic study, we evaluated the role played by ICB on MLC phosphorylation and changes in cytosolic calcium concentration in vascular smooth muscle cells.
α-Iso-cubebene (ICB) was purified from the dried fruits of Schisandra chinensis (SC) as described previously [18]. Briefly, SC (2.5 kg) fruit was dried, ground to a fine powder, and sequentially extracted at room temperature with n-hexane, chloroform (CHCl3), and methanol (MeOH). The hexane extract (308 g) was evaporated in vacuo and chromatographed on a 40 µm silica gel (J.T. Baker, Phillipsburg, NJ, USA) column (100×10 cm) by step gradient elution (0%, 5%, and 20% ethyl acetate in hexane and 5% methanol MeOH in CHCl3 to obtain 38 fractions). Fraction 1 (KH1PA, 3,689 mg) was separated on a silica gel column (100×3.0 cm) using 15% acetone in dichloromethane (CH2Cl2) to obtain nine fractions, and the second fraction (KH1PAIB, 999 mg) was separated on a silica gel column (100×3.0 cm) using 15% acetone in CH2Cl2 to yield ICB (316 mg). The purity of ICB was determined by HPLC (high-performance liquid chromatography) using a Phenomenex Luna C18 column (150×4.6 mm internal diameter; 5 µm particle size) and an acetonitrile-water-alcohol gradient at a flow rate of 1.0 ml/min and found to be >99%.
All animal procedures conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85-23, 2011 revision), and all experimental protocols were approved by the Pusan National University Institutional Animal Care and Use Committee. Wild-type control mice (C57BL/6J) were purchased from Jackson Laboratories. All animals were housed in an air-conditioned room at 22–25℃ and kept under a 12-h light/dark cycle. Food and water were provided ad libitum.
Phenylephrine hydrochloride (PE), acetylcholine chloride, and EGTA were purchased from Sigma-Aldrich (St. Louis, MO). MTT working solution was from EZ-Cytox (Daeil Laboratories, Seoul), and antibodies for MLC (sc-12896), p-MLC (sc-19848, sc-293109), and anti-β-actin (sc-47778) were from Santa Cruz Biotechnology. Fluo-3/AM was purchased from Thermo Fisher Scientific (Rockford, IL). All other chemicals were of reagent grade. The solid form of ICB was dissolved in 100% DMSO at a concentration of 100 mg/ml to produce a stock solutions and subsequently added to media as required.
C57BL/6J mice (20–25 g) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and thoracic aortas were rapidly removed. Aortic rings (2–3 mm thick) were suspended in 10-ml organ chambers filled with Krebs' solution (37℃) at a resting tension of 2 g. After an equilibration period of 90 min, aortic rings were constricted with KCl (60 mM) solution to stimulate the tissue. They were then washed with Krebs' solution to restore basic tension. After aortic rings had been allowed to equilibrate, sustained and stable contraction was induced by treating them with phenylephrine or norepinephrine. Changes in isometric tension were recorded using a force-displacement transducer (Grass FT 0.3, Quincy, MA, USA) connected to a Power Lab system 400 (ML 118, PowerLab, AD Instruments, Medford, MA, USA).
In this study, aortic rings were incubated with various concentrations of ICB for 10 min, and then phenylephrine (PE) or norepinephrine (NE) was added to induce transient vasoconstriction. The vasodilatory potency of ICB was studied using cumulative additions of ICB at concentrations of 1–10 µg/ml. Involvement of endothelium in ICB-induced relaxation was examined by comparing the relaxation magnitudes of endothelium-denuded and endothelium-intact specimens. Relaxations are expressed as percentage of relaxation of PE-induced tone.
Sprague-Dawley rats (Charles River Breeding Laboratories, Kingston, NY, USA) were sacrificed by CO2 inhalation, and then primary VSMCs obtained from thoracic aorta were cultured. Briefly, excised aortas were cut into ~1 mm2 segments, and placed as explants in a cell culture dish containing DMEM (Gibco BRL, Grand Island, NY) with 10% FBS (Gibco BRL). Cells were maintained in DMEM containing 10% FBS and antibiotic-antimycotic (Gibco BRL) at 37℃. An MTT assay was used to determine the viability of VSMCs. Briefly, cells (a total of 1×105 cells) were treated with MTT working solution (EZ-Cytox, Daeil Laboratories, Seoul, Republic of Korea), and incubated at 37℃ for 1 h. OD values of solution were obtained at a wavelength of 450 nm by ELISA.
Cytosolic calcium levels were measured as described elsewhere [19]. Briefly, VSMC was loaded with Fluo-3/AM in normal Tyrode's solution (pH 7.4) containing (in mM): 140 NaCl, 5.4 KCl, 1 MgCl2, 2 CaCl2, 5.5 glucose, and 5 HEPES. To deplete intracellular Ca2+ stores, cells were treated with 4 µM thapsigargin in Ca2+-free Tyrode's solution containing 0.2 mM ethylene glycol tetraacetic acid (EGTA). Real-time fluorescent images were captured every 10 s and analyzed using MetaFluor imaging software (Molecular Devices, Sunnyvale, CA).
VSMC lysates were prepared in ice-cold lysis buffer, and equal amounts of proteins were separated on 8–10% polyacrylamide gel under reducing conditions, and then transferred to nitrocellulose membranes (Amersham-Pharmacia Biotech, Piscataway, NJ). Membranes were blocked with 5% skim milk in TBST, incubated overnight with primary antibodies to total MLC and phosphorylated MLC (at Ser 19, Thr18, and The18/Ser19) in 5% skim milk. Blots were then washed with TBST, incubated with HRP-conjugated secondary antibody for 2 h, and developed using ECL Western blot detection reagents (Amersham). Membranes were re-blotted with anti-β-actin antibody (Santa Cruz Biotechnology), which was used as the internal control.
Results are presented as means±S.E.M. One-way analysis of variance followed by Tukey's multiple comparison test was used to determine the significances of intergroup differences. The analysis was conducted using Prism version 3.03 software (GraphPad Software, San Diego, CA, USA) and p-values <0.05 considered significant.
The contractility of aortic preparations with intact endothelium was increased dose-dependently by stimulation with phenylephrine (PE, 10−7−3×10−6 M) or norepinephrine (NE, 10−8−3×10−7 M). As shown in Fig. 1, pre-treatment with ICB (1–10 µg/ml) significantly and dose-dependently attenuated contractile responses to PE or NE. However, ICB alone did not affect the basal tension (2.0 g) of aortic rings.
To determine the effects of ICB on endothelium-dependent or nitric oxide-dependent vasodilation, we investigated the role of ICB on vascular relaxation induced by acetylcholine or sodium nitroprusside in aortic preparations with intact endothelium. As shown in Fig. 2, both acetylcholine and sodium nitroprusside markedly relaxed aortic rings pre-contracted with PE, and this relaxation was not affected by pretreating ICB. These results suggested that the inhibitory effects of ICB on vasocontraction induced by PE might not due to an endothelium-dependent mechanism.
To further investigate the involvement of vascular endothelium on the vascular action of ICB, the vasorelaxing effects of ICB were examined in endothelium-intact or -denuded aortic preparations pre-contracted by PE. As shown in Fig. 3, endothelium did not exhibit a significant influence on the vasodilatory role of ICB, suggesting ICB acted directly on vascular smooth muscle with high potency. Therefore, we subsequently investigated the mechanism underlying the vasodilatory effects of ICB using vascular smooth muscle cells (VSMC).
To determine whether ICB attenuated vascular contraction by acting on thick filament, we investigated the role of ICB on MLC phosphorylation at Ser19 and Thr18 sites of MLC, based on the facts that Ser19 and Thr18 are known as MLCK-preferred phosphorylation sites in MLC [20]. As shown in Fig. 4, although total MLC expression was unaffected by PE or NE, the phosphorylation of MLC at Ser19, Thr18, and Thr18 plus Ser19 was markedly increased in VSMC stimulated with PE or NE. However, these increases were markedly suppressed in cells treated with ICB at concentration of 10 µg/ml. On the other hand, ICB did not change basal MLC phosphorylation levels in VSMC.
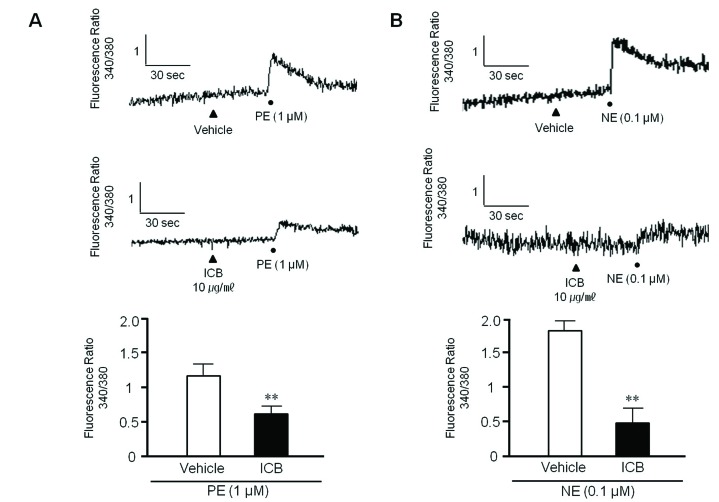
To examine whether ICB attenuates transient increases in cytosolic Ca2+ induced by PE or NE, rat VSMC were used in this study. When PE (1 µM) or NE (0.1 µM) was added to VSMC, the fluorescence emitted from cells was markedly and rapidly increased, indicating an increase in intracellular calcium concentration. However, these increases were significantly attenuated by pretreating VSMC with ICB at concentration of 10 µg/ml (Fig. 5).
Our findings show that α-iso-cubebene (ICB) attenuated vascular contraction by inhibiting the Ca2+-myosin light chain (MLC) signaling pathway. ICB attenuated cytosolic calcium increases and MLC phosphorylation in VSMC stimulated with adrenergic α-receptor agonists. In isolated aortic ring preparations, ICB significantly attenuated agonist-induced contraction, and also induced marked relaxation in pre-contracted aortic rings with or without endothelium. These observations suggested that ICB plays a pivotal role in the modulation of vascular tone by directly acting on VSMC.
Schisandra chinensis (SC) has long been used as a tonic, sedative, astringent, anti-aging agent, and as a treatment for cardiovascular symptoms in Korea, China and Japan [2122]. In our previous study, hexane extracts of SC were found to cause vasorelaxation in endothelium (ED)-intact vasculature and in ED-denuded rat thoracic aortas [16]. Furthermore, the relaxant effect of these extracts on ED-intact vasculature was more prominent than that on ED-denuded aorta [16], which suggested the importance of vascular endothelium in vascular relaxation evoked by SC extracts.
The major bioactive components of SC fruits are lignans such as ICB, schizandrins and gomisins, such as, gomisin J, N and A [2324]. Several researchers have reported the beneficial bioactivities of ICB, such as its anti-inflammatory, antiseptic and immunomodulatory activities [2526]. Also, ICB has neuroprotective [252728] and anti-inflammatory effects, the latter of which was attributed to the inhibition of endothelial expression of adhesion molecules [26]. In our previous study, ICB has been suggested to be therapeutically useful in proliferative vascular diseases by inhibiting VSMC proliferation [29]. Although ICB has been suggested to have beneficial effects on various cardiovascular symptoms, the mechanisms underlying its potential protective effects have not yet been fully investigated.
Vascular tone is an important determinant of peripheral resistance and blood pressure, and essential hypertension is characterized by abnormal increases in peripheral vascular resistance [3031]. Thus, compounds with vasodilatory effects would be expected to be useful for the treatment of vasospasm, hypertension, and the other conditions associated with hypercontractility of various vasculatures. In the present study, we investigated the effects of ICB on vascular contraction induced by adrenergic α-receptor agonists to determine the role played by ICB on the modulation of vascular tone. In isolated preparations of mouse thoracic aorta, ICB dose-dependently attenuated the vasocontraction induced by PE or NE. Vascular contraction in response to PE is known to involve Ca2+ release from intracellular stores, and Ca2+ movement into cytosol due to store-operated Ca2+ influx and/or receptor operated Ca2+ channels [3233]. However, it was reported in a recent study in which PE failed to elicit significant intracellular Ca2+ release [34], thus it would appear that PE- or NE-induced vascular contraction occurs as a result of Ca2+ influx through receptor operated channels and voltage-dependent channels [3435].
In the present study, ICB attenuated increases in cytosolic calcium levels in VSMC stimulated with adrenergic α-agonists. Moreover, the contraction induced by PE and NE in isolated aortic rings of mice was markedly attenuated by ICB, whereas ICB has no effects on high K+ (60 mM)-induced vasocontraction. Because high K+-induced vasocontraction is mediated by membrane depolarization of VSMC through an opening of voltage-gated Ca2+ channels [36], these observations suggested that the inhibitory effects of ICB on α-agonist-induced vasocontraction might be due, at least in part, to Ca2+ influx through receptor operated channels, but not to voltage-dependent channels.
Several intracellular mechanisms are involved in the modulation of vascular tone, and increased cytoplasmic Ca2+ and phosphorylation of MLC might be the most crucial factors [5]. MLC phosphorylation is determined by the relative activities of Ca2+-dependent myosin light chain kinase, and is directly linked with smooth muscle contraction [6]. Furthermore, several studies have shown that MLC phosphatase also significantly contributes to MLC phosphorylation [81037]. In the case of vascular smooth muscle, contractile agonists such as adrenergic α-agonists increase force generation even when Ca2+concentration remain constant, in which MLC phosphatase plays an important role [1137]. In the present study, we evaluated the role of ICB on MLC phosphorylation in VSMC as a mechanistic study. Among various sites in MLC including Ser1, Ser8, ser19, Thr9, and Thr18, Ser19 and Thr18 are known as MLCK-preferred phosphorylation sites [20]. Thus, we identified the effects of ICB on MLC phosphorylation at Ser19 and Thr18 sites. In the VSMC, adrenergic α-agonists including PE and NE increase MLC phosphorylation in association with an increases in cytosolic calcium. Both MLC phosphorylation and changes in cytosolic calcium induced by PE or NE were significantly attenuated by ICB. Thus, it was suggested that ICB regulated vascular tone by inhibiting MLC phosphorylation and calcium flux. However, further studies are required to clarify the detail signaling mechanism, in which ICB regulates MLC phosphorylation.
Arterial hypertension is one of the most common cardiovascular disorders and is characterized by altered vascular tone and increased vascular contractility resulting in high blood pressure [383940]. In our present study, the contractile agonists such as PE and NE provoked Ca2+ mobilization and increased cytosolic Ca2+, which in turn increased MLC phosphorylation, thus increasing vascular contraction. Furthermore, the vascular contraction induced by adrenergic α-agonists was attenuated by ICB via the inhibition of MLC phosphorylation and cytosolic Ca2+ fluxes into VSMC. These results encourage us to suggest that ICB is considered as a potential therapeutic intervention for the treatment of cardiovascular disorders, such as cerebral and coronary vasospasm, hypertension, and the other conditions associated with hypercontractility of the various vasculatures.
ACKNOWLEDGEMENTS
This work was supported by a 2-Year Research Grant of Pusan National University, Republic of Korea.
Notes
References
1. Flack JM, Oparil S, Pratt JH, Roniker B, Garthwaite S, Kleiman JH, Yang Y, Krause SL, Workman D, Saunders E. Efficacy and tolerability of eplerenone and losartan in hypertensive black and white patients. J Am Coll Cardiol. 2003; 41:1148–1155. PMID: 12679215.

2. Ohanian J, Ohanian V. Lipid second messenger regulation: the role of diacylglycerol kinases and their relevance to hypertension. J Hum Hypertens. 2001; 15:93–98. PMID: 11317187.

3. Touyz RM, He G, Wu XH, Park JB, Mabrouk ME, Schiffrin EL. Src is an important mediator of extracellular signal-regulated kinase 1/2-dependent growth signaling by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients. Hypertension. 2001; 38:56–64. PMID: 11463760.

4. Staiculescu MC, Galiñanes EL, Zhao G, Ulloa U, Jin M, Beig MI, Meininger GA, Martinez-Lemus LA. Prolonged vasoconstriction of resistance arteries involves vascular smooth muscle actin polymerization leading to inward remodelling. Cardiovasc Res. 2013; 98:428–436. PMID: 23417038.

5. Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev. 1996; 76:967–1003. PMID: 8874491.

6. Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994; 372:231–236. PMID: 7969467.

7. Kim JI. High fat diet confers vascular hyper-contractility against angiotensin II through upregulation of MLCK and CPI-17. Korean J Physiol Pharmacol. 2017; 21:99–106. PMID: 28066146.

8. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and non-muscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003; 83:1325–1358. PMID: 14506307.
9. Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003; 27:201–206. PMID: 14627618.

10. Christ G, Wingard C. Calcium sensitization as a pharmacological target in vascular smooth-muscle regulation. Curr Opin Investig Drugs. 2005; 6:920–933.
11. Kitazawa T, Gaylinn BD, Denney GH, Somlyo AP. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991; 266:1708–1715. PMID: 1671041.
12. Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP, Somlyo AV. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J Biol Chem. 1992; 267:14662–14668. PMID: 1321813.

13. Mu Y, Zhang J, Zhang S, Zhou HH, Toma D, Ren S, Huang L, Yaramus M, Baum A, Venkataramanan R, Xie W. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J Pharmacol Exp Ther. 2006; 316:1369–1377. PMID: 16267138.

14. Huang SX, Li RT, Liu JP, Lu Y, Chang Y, Lei C, Xiao WL, Yang LB, Zheng QT, Sun HD. Isolation and characterization of biogenetically related highly oxygenated nortriterpenoids from Schisandra chinensis. Org Lett. 2007; 9:2079–2082. PMID: 17480084.

15. Rhyu MR, Kim EY, Yoon BK, Lee YJ, Chen SN. Aqueous extract of Schizandra chinensis fruit causes endothelium-dependent and -independent relaxation of isolated rat thoracic aorta. Phytomedicine. 2006; 13:651–657. PMID: 16704926.

16. Park JY, Shin HK, Lee YJ, Choi YW, Bae SS, Kim CD. The mechanism of vasorelaxation induced by Schisandra chinensis extract in rat thoracic aorta. J Ethnopharmacol. 2009; 121:69–73. PMID: 18983904.

17. Park JY, Choi YW, Yun JW, Bae JU, Seo KW, Lee SJ, Park SY, Kim CD. Gomisin J from Schisandra chinensis induces vascular relaxation via activation of endothelial nitric oxide synthase. Vascul Pharmacol. 2012; 57:124–130. PMID: 22728282.

18. Lee YJ, Shim JW, Lee YJ, Park YH, Lee HY, Kim SD, Choi YW, Bae YS. Identification of a novel compound that stimulates intracellular calcium increase and CXCL8 production in human neutrophils from Schisandra chinensis. Biochem Biophys Res Commun. 2009; 379:928–932. PMID: 19138665.

19. Qi H, Li L, Shuai J. Optimal microdomain crosstalk between endoplasmic reticulum and mitochondria for Ca2+ oscillations. Sci Rep. 2015; 5:7984. PMID: 25614067.

20. Turbedsky K, Pollard TD, Bresnick AR. A subset of protein kinase C phosphorylation sites on the myosin II regulatory light chain inhibits phosphorylation by myosin light chain kinase. Biochemistry. 1997; 36:2063–2067. PMID: 9047304.

21. Chen K, Li C. Recent advances in studies on traditional Chinese anti-aging material medica. J Tradit Chin Med. 1993; 13:223–226. PMID: 8246603.
22. You JS, Pan TL, Hou YC. Schisandra chinensis protects against adriamycin-induced cardiotoxicity in rats. Chang Gung Med J. 2006; 29:63–70. PMID: 16642728.
23. Choi YW, Takamatsu S, Khan SI, Srinivas PV, Ferreira D, Zhao J, Khan IA. Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: structure-antioxidant activity relationships of dibenzocyclooctadiene lignans. . J Nat Prod. 2006; 69:356–359. PMID: 16562834.

24. Lu Y, Chen DF. Analysis of Schisandra chinensis and Schisandra sphenanthera. J Chromatogr A. 2009; 1216:1980–1990. PMID: 18849034.

25. Park SY, Park SJ, Park NJ, Joo WH, Lee SJ, Choi YW. α-Isocubebene exerts neuroprotective effects in amyloid beta stimulated microglia activation. Neurosci Lett. 2013; 555:143–148. PMID: 24090820.

26. Choi YW, Kim HJ, Park SS, Chung JH, Lee HW, Oh SO, Kim BS, Kim JB, Chung HY, Yu BP, Kim CD, Yoon S. Inhibition of endothelial cell adhesion by the new anti-inflammatory agent alpha-isocubebene. Vascul Pharmacol. 2009; 51:215–224. PMID: 19539051.
27. Park SY, Park TG, Lee SJ, Bae YS, Ko MJ, Choi YW. α-Iso-cubebenol inhibits inflammation-mediated neurotoxicity and amyloid beta 1-42 fibril-induced microglial activation. J Pharm Pharmacol. 2014; 66:93–105. PMID: 24138316.

28. Park SY, Choi YH, Park G, Choi YW. Neuroprotective effects of α-iso-cubebenol on glutamate-induced neurotoxicity. Environ Toxicol Pharmacol. 2015; 40:549–556. PMID: 26322719.

29. Jang MA, Lee SJ, Baek SE, Park SY, Choi YW, Kim CD. α-Iso-Cubebene inhibits PDGF-induced vascular smooth muscle cell proliferation by suppressing osteopontin expression. PLoS One. 2017; 12:e0170699. PMID: 28114367.

30. Safar ME, Balkau B, Lange C, Protogerou AD, Czemichow S, Blacher J, Levy BI, Smulyan H. Hypertension and vascular dynamics in men and women with metabolic syndrome. J Am Coll Cardiol. 2013; 61:12–19. PMID: 23287369.

31. Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P) H oxidase-sensitive pathways. J Hypertens. 2001; 19:1245–1254. PMID: 11446714.
32. Nishimura J, Khalil RA, van Breemen C. Agonist-induced vascular tone. Hypertension. 1989; 13:835–844. PMID: 2544524.

33. Sun N, Hong T, Zhang R, Yang X. The effects of verapamil SR and bisoprolol on reducing the sympathetic nervous system's activity. Hypertens Res. 2000; 23:537–540. PMID: 11016810.

34. Zheng C, Lo CY, Meng Z, Li Z, Zhong M, Zhang P, Lu J, Yang Z, Yan F, Zhang Y, Huang Y, Yao X. Gastrodin inhibits store-operated Ca2+ entry and alleviates cardiac hypertrophy. Front Pharmacol. 2017; 8:222. PMID: 28487655.

35. Meisheri KD, Hwang O, van Breemen C. Evidence for two separated Ca2+ pathways in smooth muscle plasmalemma. J Membr Biol. 1981; 59:19–25. PMID: 7241573.
36. Eto K, Ohya Y, Nakamura Y, Abe I, Fujishima M. Comparative actions of insulin sensitizers on ion channels in vascular smooth muscle. Eur J Pharmacol. 2001; 423:1–7. PMID: 11438300.

37. Shibata K, Sakai H, Huang Q, Kamata H, Chiba Y, Misawa M, Ikebe R, Ikebe M. Rac1 regulates myosin II phosphorylation through regulation of myosin light chain phosphatase. J Cell Physiol. 2015; 230:1352–1364. PMID: 25502873.

38. Kokubo Y, Okamura T, Watanabe M, Higashiyama A, Ono Y, Miyamoto Y, Furukawa Y, Kamide K, Kawanishi K, Okayama A, Yoshimasa Y. The combined impact of blood pressure category and glucose abnormality on the incidence of cardiovascular diseases in a Japanese urban cohort: the Suita Study. Hypertens Res. 2010; 33:1238–1243. PMID: 20927111.

39. Seok YM, Cho HJ, Cha BY, Woo JT, Kim IK. Honokiol attenuates vascular contraction through the inhibition of the RhoA/Rhokinase signalling pathway in rat aortic rings. J Pharm Pharmacol. 2011; 63:1244–1251. PMID: 21827498.

40. Kang G, Lee YR, Joo HK, Park MS, Kim CS, Choi S, Jeon BH. Trichostatin A modulates angiotensin II-induced vasoconstriction and blood pressure via inhibition of p66shc activation. Korean J Physiol Pharmacol. 2015; 19:467–472. PMID: 26330760.

Fig. 1
Effects of α-isocubebene (ICB) on phenylephrine (PE)- and norepinephrine (NE)-induced contractions in mouse thoracic aorta.
Under pretreatment with vehicle or ICB (1–10 µg/ml), PE (10−7−3×10−6 M) (A) or NE (10−8−3×10−7 M) (B) was added in a step-wise manner to elicit vasocontraction. Tracings are representative of 4–5 independent experiments. Bottom: percentage vasocontraction represents contraction relative to that elicited by 60 mM KCl. Results were presented as the means±SEM of 4–5 independent experiments. *p<0.05; **p<0.01 vs. corresponding value in Vehicle.
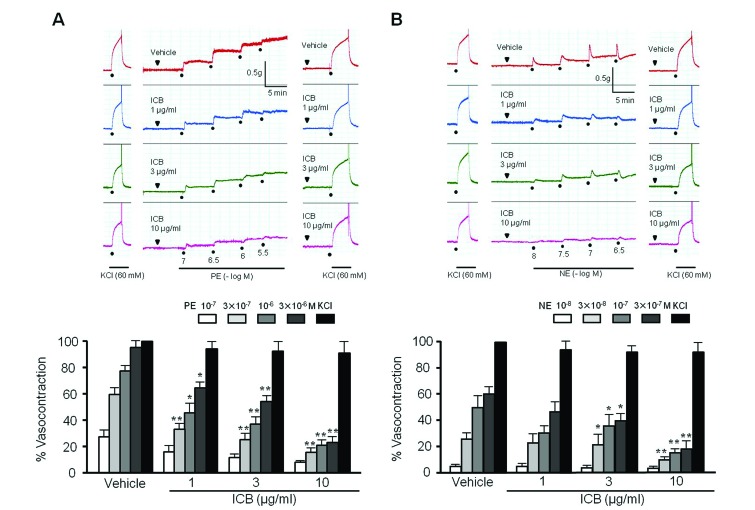
Fig. 2
Effects of α-isocubebene (ICB) on acetylcholine (Ach)- or sodium nitroprusside (SNP)-induced vasodilation in mouse thoracic aorta pre-contracted with phenylephrine (PE, 1 µM).
Under pretreatment with vehicle or ICB (1–10 µg/ml), Ach (1 µM) or SNP (0.1 µM) was added to elicit vasodilation. Tracings are representative of 5 independent experiments (A). Percentage vasodilation was presented as the means±SEM of 5 independent experiments (B).
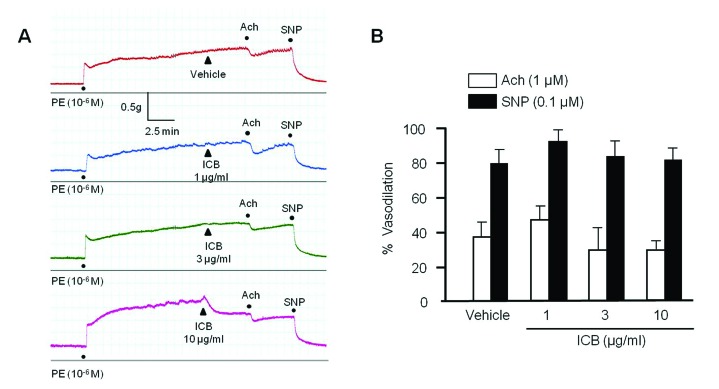
Fig. 3
Effects of α-isocubebene (ICB) on vascular tension in mouse thoracic aorta pre-contracted with phenylephrine (PE, 1 µM).
In mice thoracic
aorta with (ED+) or without (ED−) endothelium, ICB (3–30 µg/ml) was added cumulatively to elicit vasodilation. Tracings are representative of 6 independent experiments (A). Percentage vasodilation represents relative value to vasodilation induced by SNP (0.1 µM). Data was presented as the means±SEM of 6 independent experiments (B).
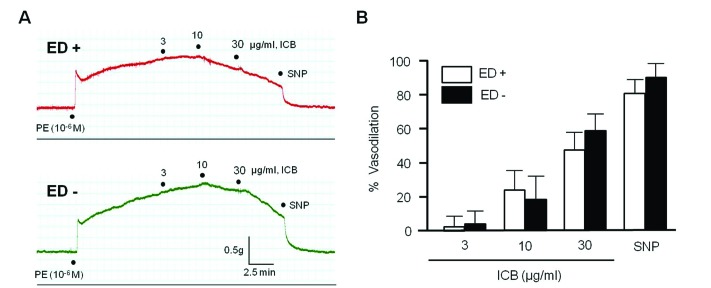
Fig. 4
Inhibitory effect of α-isocubebene (ICB) on MLC phosphorylation in phenylephrine (PE) (A)- or norepinephrine (NE) (B)-treated VSMC.
After pretreating cells with vehicle or ICB (10 µg/ml), PE (1 µM) or NE (1 µM) was added to elicit MLC phosphorylation. Blots are representative of 5–6 independent experiments. Densitometric results of Western blot are shown in the bottom panel. The p-MLC to t-MLC ratios were presented as the means±SEM of 5–6 independent experiments. **p<0.01 vs. corresponding value in control. ##p<0.01 vs. corresponding value in vehicle.
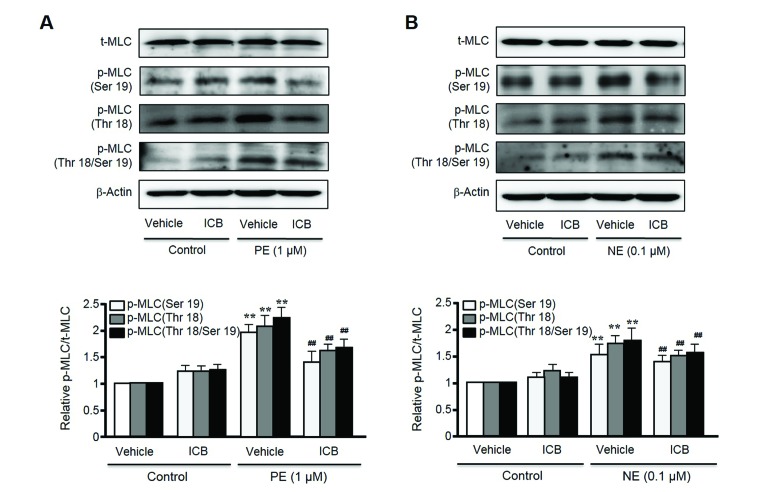
Fig. 5
Effects of α-isocubebene (ICB) on cytosolic Ca2+ levels in vascular smooth muscle cells.
After pretreating cells with vehicle or ICB (10 µg/ml), phenylephrine (PE, 1 µM) (A) or norepinephrine (NE, 0.1 µM) (B) was added to induce calcium influx. Tracings are representative of 4–5 independent experiments (A). Bottom panel: Relative fluorescence ratio was presented as the means±SEM of 4–5 independent experiments. **p<0.01 vs. vehicle.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download