Abstract
The superficial dorsal horn of the spinal cord plays an important role in pain transmission and opioid activity. Several studies have demonstrated that opioids modulate pain transmission, and the activation of µ-opioid receptors (MORs) by opioids contributes to analgesic effects in the spinal cord. However, the effect of the activation of MORs on GABAergic interneurons and the contribution to the analgesic effect are much less clear. In this study, using transgenic mice, which allow the identification of GABAergic interneurons, we investigated how the activation of MORs affects the excitability of GABAergic interneurons and synaptic transmission between primary nociceptive afferent and GABAergic interneurons. We found that a selective µ-opioid agonist, [D-Ala2, NMe-Phe4, Gly-ol]-enkephanlin (DAMGO), induced an outward current mediated by K+ channels in GABAergic interneurons. In addition, DAMGO reduced the amplitude of evoked excitatory postsynaptic currents (EPSCs) of GABAergic interneurons which receive monosynaptic inputs from primary nociceptive C fibers. Taken together, we found that DAMGO reduced the excitability of GABAergic interneurons and synaptic transmission between primary nociceptive C fibers and GABAergic interneurons. These results suggest one possibility that suppression of GABAergic interneurons by DMAGO may reduce the inhibition on secondary GABAergic interneurons, which increase the inhibition of the secondary GABAergic interneurons to excitatory neurons in the spinal dorsal horn. In this circumstance, the sum of excitation of the entire spinal network will control the pain transmission.
It is well known that a majority of the nociceptive information is transferred to the superficial dorsal horn of the spinal cord by Aδ and C primary afferent fibers which interact with spinal dorsal horn neurons [123]. Nociceptive information from primary afferent fibers is integrated in the superficial dorsal horn and further transmitted to higher levels in the pain transmission pathway. The superficial dorsal horn can be divided into lamina I and II. Lamina II neurons, the substantia gelatinosa (SG) neurons, are mainly involved in pain transmission and interact with nociceptive fibers (Aδ, C) [14]. SG neurons are heterogeneous and comprise excitatory neurons and inhibitory interneurons. Inhibitory (GABAergic) interneurons account for about 30–40% of SG neurons [56789] and these GABAergic interneurons receive monosynaptic nociceptive Aδ or C fiber inputs and non-nociceptive Aβ inputs [10111213]. These GABAergic interneurons play an important role in pain processing. Dorsal horn neurons normally receive an inhibitory control of the GABAergic interneurons. Loss of these GABAergic interneurons in the spinal dorsal horn due to peripheral nerve injury contributes to reduced GABAergic tone and further enhances central sensitization [1415]. Thus, identifying the role of GABAergic interneurons in spinal circuits is crucial to understanding the mechanism of pain processing.
Superficial dorsal horn of the spinal cord is also involved in the opioid activity in rodents, cats and humans [1617181920]. Several studies have demonstrated that opioids modulate synaptic transmission in pain pathways and produce analgesic effects. Three different types of classical receptors are activated by opioids namely µ, δ and κ-opioid receptors [21]. Among these, the µ-opioid receptor (MOR) plays an important role in analgesia and is widely expressed at various levels in the central nervous system associated with pain transmission [22]. In particular, a lot of MORs are distributed in the spinal cord, especially in the central terminals of primary afferents and dorsal horn neurons [222324]. MORs can reduce neuronal activity through various presynaptic and postsynaptic mechanisms [2526]. Activation of MORs by [D-Ala2, NMe-Phe4, Gly-ol]-enkephanlin (DAMGO), a selective µ-opioid agonist, reduces the excitability of SG neurons [17182728]. Previous studies have reported that intrathecal administration of DAMGO suppressed nociceptive activity in the spinal dorsal horn [19]. The activation of MORs also causes behavioral analgesia associated with inhibition of spinal nociceptive neurons [29].
Previous studies investigating the effects of MOR activation in the spinal dorsal horn have been limited to unidentified neurons, since it was difficult to distinguish whether the recorded ones are excitatory or inhibitory neurons. Thus, despite the accumulated research demonstrating the effects of MORs in modulating pain processing, the mechanism of this modulation in spinal circuits is not yet clear. Given the role of GABAergic interneurons in pain control, it is important to understand how these interneurons are regulated by the activation of MORs. In this study, using transgenic mice that allow GABAergic interneuron identification, we investigated the effect of MOR activation by DAMGO in GABAergic interneurons which directly interact with primary nociceptive C fibers. Our observation suggests that DAMGO reduce the activity of GABAergic interneurons and the analgesic action of DAMGO is the result of total sum of excitation and inhibition throughout the entire spinal neural network.
All experiments procedures were approved by the Seoul National University Institutional Animal Care and Use Committee and performed in accordance with the guidelines of the National Institutes of Health. As our previous research reported [11], to identify GABAergic interneurons, we used heterozygous C57BL/6J BAC transgenic mice expressing enhanced green fluorescence protein (eGFP) under the control of the GAD65 promoter. The vivarium was controlled with 12 h light/dark cycle and all experiments were performed during the daylight hours.
To prepare the spinal lumbar slices, we used 4-weeks-old transgenic mice. The mice were anesthetized with 10% urethane (i.p. injection, 1.5 mg/kg) and a laminectomy was performed after enough anesthesia was confirmed. During the laminectomy, the spinal cord from each mouse was extracted and quickly transferred to the ice-cold cutting solution consisted of (in mM) 95 NaCl, 1.8 KCl, 1.2 KH2PO4, 0.5 CaCl2, 7 MgSO4, 26 NaHCO3, 15 glucose, and 50 sucrose, oxygenated with 95% O2 and 5% CO2, at a pH of 7.35–7.45 and an osmolarity of 310–320 mOsm to cut lumbar segment. From the lumbar segment, transverse spinal slices with L4 or L5 dorsal roots were prepared using a microm HM 650V vibratome (Walldorf, Germany), and the slices were incubated at 34℃ for 30 min for a recovery.
The slice was transferred to the recording chamber and continuously superfused with artificial cerebrospinal fluid (aCSF) consisted of (in mM) 124 NaCl, 2.5 KCl, 1 KH2PO4, 2.5 CaCl2, 1.3 MgSO4, 26.2 NaHCO3, and 20 glucose, oxygenated with 95% O2 and 5% CO2, at a pH of 7.35–7.45 and an osmolarity of 300–310 mOsm. Picrotoxin (100 µM) and strychnine (4 µM) were included in the aCSF to block inhibitory synaptic transmissions. Substantia gelatinosa (SG) neurons were identified as a translucent band across the spinal dorsal horn and were visualized with an Olympus BX 50WI microscope (Olympus Optical, Tokyo, Japan) and a 60x water-immersion objective. Glass pipettes were filled with the internal solution consisted of (in mM) 120 K-gluconate, 10 KCl, 2 MgATP, 0.5 NaGTP, 20 HEPES, 0.5 EGTA, and 10 phosphocreatine di (tris) salt at a pH of 7.29 and an osmolarity of 300 mOsm and the resistance of the pipettes was 3–5 MΩ. All experiments in this study were acquired using multiclamp 700B patch-clamp amplifier (Axon Instruments), and the signals were filtered at 2 kHz. To record evoked excitatory postsynaptic currents (EPSCs) of GABAergic interneurons in SG, the attached dorsal root was stimulated through a suction electrode with a constant current stimulator (A360; WPI) at 0.1 Hz. To confirm whether the recorded SG neurons directly receive excitatory input from primary nociceptive C fibers, the latency was calculated. If the latency was unchanged and failures did not occur by stimulation of C fibers at 1 Hz, these neurons were considered as directly interacting with primary nociceptive C fibers. Nociceptive Aδ and C fibers are well distinguished based upon their stimulation thresholds (Aδ, 0/05–0.30 mA; C, 0.8–2.0 mA) and estimated conduction velocity (Aδ fiber, 2–8 m/s; C fiber, <2 m/s) [113031].
All drugs (DAMGO, picrotoxin, strychnine) were purchased from Sigma. We used DAMGO with its concentration of 1 µM, picrotoxin in 100 µM and strychnine as 4 µM when they present in the in aCSF solution.
Data were analyzed using Igor and plotted using Prism 7. All data are represented as mean±s.e.m. Two-way RM ANOVA and Two-tailed paired t-test were used to determine the significance in statistical comparisons. The differences were considered significant if p value is below 0.05. NS indicates p>0.05.
All recorded neurons were obtained from lamina II of the spinal dorsal horn. To confirm whether recorded GABAergic neurons directly receive excitatory inputs from nociceptive C fibers, we recorded evoked excitatory postsynaptic currents (EPSCs) of GABAergic interneurons while stimulating the attacheddorsal root, and the latency to produce EPSCs was calculated. Fig. 1A shows a representative neuron which receives monosynaptic C primary afferent inputs. As the latency was constant and its failure did not occur by the stimulation of C fibers at 1 Hz, this neuron can be considered as directly synapses with primary nociceptive C fibers. All recordings were performed on these neurons which formed synapses directly with C fibers.
We first investigated the effect of DAMGO on the excitability of GABAergic interneurons. In 70% of GABAergic interneurons examined (n=30), an outward current was produced by superfusing DAMGO (1 µM) for 2 min. This outward current was reversible and reproducible when DAMGO was repeatedly administered (39.55±2.16 pA, 4 trials, Fig. 1B). This result indicates that GABAergic interneurons were hyperpolarized by DAMGO treatment and neuronal excitability was reduced.
Next, we examined which channels mediate the DAMGO-induced current. In voltage clamp mode at −70 mV, membrane currents of GABAergic interneurons were recorded by applying voltage command steps in the absence and presence of DAMGO. Voltage steps were commanded from −120 mV to −30 mV with 10 mV steps each lasting 500 ms. Fig. 2A shows representative traces of varying current responses from GABAergic interneurons in the absence and presence of DAMGO. DAMGO-induced current is significantly higher during DAMGO treatment than that during normal aCSF. In current-voltage (I-V) relationship, reversal potential of DAMGO-induced current was close to −80 mV and exhibited inward rectification properties (rectification index (RI)=9.47±2.49, n=6, Fig. 2B). The following experiments were conducted to determine whether the DAMGO-induced current is related to K+ channel. In voltage clamp mode at −70 mV, membrane currents of GABAergic interneurons were recorded by applying voltage command steps in the absence and presence of Ba2+, a K+ channel blocker. The DAMGO-induced current was completely blocked and did not occur under Ba2+ (Fig. 2C). We also verified that DAMGO-induced current increased when the concentration of K+ was high in the extracellular solution (Fig. 3). Fig. 3A shows representative traces of current responses at normal (2.5 mM) and high (8.5 mM) concentrations of K+ in the external solution during DAMGO treatment. As shown in Fig. 3B, the DAMGO-induced current significantly increased at high K+ concentrations (n=5). Taken together, we conclude that DAMGO-induced current of GABAergic interneurons is mediated by K+ channels.
To further examine the effect of DAMGO on excitatory synaptic transmission from primary nociceptive C fibers to GABAergic interneurons in SG, we recorded evoked EPSCs (eEPSCs) in GABAergic interneurons by attached dorsal root stimulation at a holding potential (Vh) of −70 mV. We verified whether the targeted neurons receive monosynaptic inputs from primary nociceptive C fibers using the procedure described in Fig. 1A. As shown in Fig. 4, DAMGO significantly reduced the amplitude of eEPSCs by 42.24±10.41% of controls and this effect of DAMGO on the amplitude recovered by 9.92±7.02% over a period of 10 min (Fig. 4B). This result suggests that DAMGO reduced excitatory synaptic input from primary afferents to GABAergic interneurons of spinal dorsal horn.
In this study, we investigated how activation of MORs affects spinal GABAergic interneurons which receive monosynaptic inputs from primary nociceptive fibers. The µ-opioid agonist DAMGO induces an outward current in GABAergic interneurons. The DAMGO-induced current is mediated by K+ channels because the current was completely blocked by application of Ba2+ solution, a K+ channel blocker and exhibited inward rectification properties. We also showed that the amplitude of eEPSCs in GABAergic interneurons was reduced by DAMGO, indicating that activation of MORs reduces excitatory synaptic transmission between primary nociceptive C fibers and GABAergic interneurons in the spinal dorsal horn.
Opioids are well known to exert an analgesic effect by acting on both presynaptic and postsynaptic MORs within the spinal dorsal horn [182325262832]. Opioids bind directly to MORs on primary afferent fibers, resulting in a decrease in excitatory neurotransmitter release from primary afferent fibers. In addition, opioids act on dorsal horn neurons leading to a reduction in the excitability of these neurons. MORs activated by opioids are inhibitory G-coupled protein receptors (Gi) that are coupled to inwardly rectifying K+ (GIRK) channels. Activation of MORs results in the opening of K+ channels and reduces the excitability of neurons. As a result of these mechanisms, opioids can suppress pain transmission within the spinal cord. Several electrophysiological studies have demonstrated that DAMGO-induced outward currents are mediated by GIRK channels and exhibit inward rectification in the spinal cord of rodents and cats [1733]. However, these accumulated findings has been limited to unidentified neurons which may either be excitatory or inhibitory. Several studies using immunoreactivity reported that a minority of neurons expressing MORs contains GABA [34]. And GIRK channels coupled to MORs are mainly found in excitatory neurons in the spinal dorsal horn [24]. Here, we identified GABAergic interneurons using transgenic mice expressing green fluorescent protein (GFP) and examined the effect of DAMGO on these interneurons. In our study, outward currents was produced in GABAergic interneurons by DAMGO treatment and these neurons exhibited inwardly rectifying I-V relationship, which imply that GIRK channels are activated by DAMGO in GABAergic interneurons. In addition, DAMGO can act on MORs expressed in presynaptic primary afferents. Consistent with this result, reduced excitatory synaptic transmission between primary nociceptive afferent and GABAergic interneurons shows the possibility.
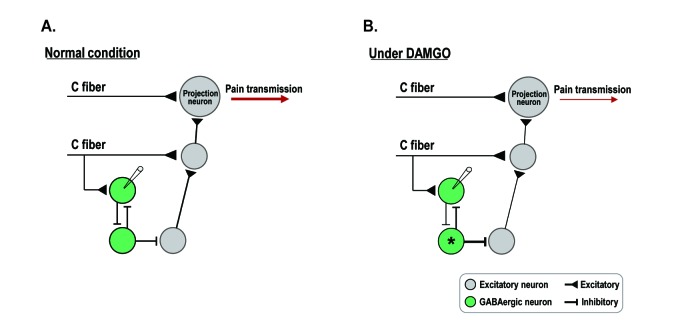
In this study, the excitability of GABAergic interneurons was reduced by DAMGO treatment. This seems to lead to increased pain transmission as a result of the absence of inhibitory control by GABAergic interneurons [1415]. Given that the activity of opioids in the spinal cord produces analgesia, our result may appear inconsistent with the already known analgesic effects of DAMGO. However, as shown in Fig. 5, spinal circuits are complicated by excitatory neurons, inhibitory interneurons and projection neurons [5]. Previous studies reported that GABAergic interneurons are interconnected and inhibit each other as well as excitatory neurons [1235]. In our study, the excitability and synaptic transmission of GABAergic interneurons which directly interact with primary nociceptive C fibers were reduced by DAMGO treatment. These results suggest one possibility that suppression of the GABAergic interneurons by DAMGO may reduce the inhibition on secondary GABAergic interneurons. If so, the inhibition of GABAergic interneurons (*) toward excitatory neurons can be enhanced and finally reduced pain transmission (Fig. 5B). In addition, DAMGO does not act on specific cell populations to exert analgesic effects, but on the entire spinal neural network, including MORs expressed in primary afferent fibers and excitatory neurons in the spinal dorsal horn. Therefore, a possible explanation for our findings is that, the analgesic action of DAMGO is the result of the total sum of excitation and inhibition throughout the entire spinal neural network.
In this study, we propose a possible mechanism to explain how DAMGO induces analgesic effects in spinal dorsal horn. Our experiments were limited to GABAergic interneurons expressing eGFP under the control of the glutamate acid decarboxylase (GAD) 65 promoter. Considering that several subtypes of interneurons coexist in spinal circuits, further studies are required to provide a deeper understanding of how opioids act as analgesics in the spinal cord using dual patch recording or Ca2+ imaging which permit simultaneous recording of multiple interneurons.
ACKNOWLEDGEMENTS
This work was supported by grants from National Research Foundation of Korea (2012R1A5A2A44671346 and BK21 Plus Project).
Notes
References
1. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009; 139:267–284. PMID: 19837031.

2. Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986; 234:358–361. PMID: 3764416.

3. Kumazawa T, Perl ER. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978; 177:417–434. PMID: 412881.

4. Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979; 186:117–131. PMID: 447880.

5. Zeilhofer HU, Wildner H, Yévenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012; 92:193–235. PMID: 22298656.

6. Spike RC, Todd AJ, Johnston HM. Coexistence of NADPH diaphorase with GABA, glycine, and acetylcholine in rat spinal cord. J Comp Neurol. 1993; 335:320–333. PMID: 8227522.

7. Todd AJ, McKenzie J. GABA-immunoreactive neurons in the dorsal horn of the rat spinal cord. Neuroscience. 1989; 31:799–806. PMID: 2594201.

8. Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990; 296:496–505. PMID: 2358549.

9. Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I-III of the mammalian spinal dorsal horn. Prog Neurobiol. 1993; 41:609–645. PMID: 7904359.

10. Furue H, Katafuchi T, Yoshimura M. Sensory processing and functional reorganization of sensory transmission under pathological conditions in the spinal dorsal horn. Neurosci Res. 2004; 48:361–368. PMID: 15041189.

11. Cui L, Kim YR, Kim HY, Lee SC, Shin HS, Szabó G, Erdélyi F, Kim J, Kim SJ. Modulation of synaptic transmission from primary afferents to spinal substantia gelatinosa neurons by group III mGluRs in GAD65-EGFP transgenic mice. J Neurophysiol. 2011; 105:1102–1111. PMID: 21177998.

12. Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003; 23:8752–8758. PMID: 14507975.

13. Yasaka T, Kato G, Furue H, Rashid MH, Sonohata M, Tamae A, Murata Y, Masuko S, Yoshimura M. Cell-type-specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal horn in vitro. J Physiol. 2007; 581:603–618. PMID: 17347278.
14. Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GAB-Aergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002; 22:6724–6731. PMID: 12151551.

15. Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006; 26:1833–1843. PMID: 16467532.

16. Fürst S. Transmitters involved in antinociception in the spinal cord. Brain Res Bull. 1999; 48:129–141. PMID: 10230704.

17. Cho PS, Lee HK, Lee SH, Im JZ, Jung SJ. DAMGO modulates two-pore domain K+ channels in the substantia gelatinosa neurons of rat spinal cord. Korean J Physiol Pharmacol. 2016; 20:525–531. PMID: 27610039.
18. Wu SY, Ohtubo Y, Brailoiu GC, Dun NJ. Effects of endomorphin on substantia gelatinosa neurons in rat spinal cord slices. Br J Pharmacol. 2003; 140:1088–1096. PMID: 14530213.

19. Omote K, Kitahata LM, Collins JG, Nakatani K, Nakagawa I. The antinociceptive role of mu- and delta-opiate receptors and their interactions in the spinal dorsal horn of cats. Anesth Analg. 1990; 71:23–28. PMID: 1973027.
20. Czlonkowski A, Costa T, Przewlocki R, Pasi A, Herz A. Opiate receptor binding sites in human spinal cord. Brain Res. 1983; 267:392–396. PMID: 6307472.
21. Chang KJ, Cuatrecasas P. Multiple opiate receptors. Enkephalins and morphine bind to receptors of different specificity. J Biol Chem. 1979; 254:2610–2618. PMID: 218947.

22. Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995; 15:3328–3341. PMID: 7751913.

23. Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990; 521:15–22. PMID: 2169958.
24. Marker CL, Luján R, Colón J, Wickman K. Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels. J Neurosci. 2006; 26:12251–12259. PMID: 17122050.

25. Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000; 40:389–430. PMID: 10836142.

26. Yaksh TL. Pharmacology and mechanisms of opioid analgesic activity. Acta Anaesthesiol Scand. 1997; 41:94–111. PMID: 9061092.

27. Glaum SR, Miller RJ, Hammond DL. Inhibitory actions of delta 1-, delta 2-, and mu-opioid receptor agonists on excitatory transmission in lamina II neurons of adult rat spinal cord. J Neurosci. 1994; 14:4965–4971. PMID: 8046463.

28. Kohno T, Kumamoto E, Higashi H, Shimoji K, Yoshimura M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J Physiol. 1999; 518:803–813. PMID: 10420016.

29. Yaksh TL. Opiate receptors for behavioral analgesia resemble those related to the depression of spinal nociceptive neurons. Science. 1978; 199:1231–1233. PMID: 204008.

30. Chen J, Sandkühler J. Induction of homosynaptic long-term depression at spinal synapses of sensory a delta-fibers requires activation of metabotropic glutamate receptors. Neuroscience. 2000; 98:141–148. PMID: 10858620.
31. Ruscheweyh R, Sandkühler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. J Physiol. 2002; 541:231–244. PMID: 12015432.
32. Kerchner GA, Zhuo M. Presynaptic suppression of dorsal horn inhibitory transmission by mu-opioid receptors. J Neurophysiol. 2002; 88:520–522. PMID: 12091574.
33. Schneider SP, Eckert WA 3rd, Light AR. Opioid-activated postsynaptic, inward rectifying potassium currents in whole cell recordings in substantia gelatinosa neurons. J Neurophysiol. 1998; 80:2954–2962. PMID: 9862898.

34. Kemp T, Spike RC, Watt C, Todd AJ. The mu-opioid receptor (MOR1) is mainly restricted to neurons that do not contain GABA or glycine in the superficial dorsal horn of the rat spinal cord. Neuroscience. 1996; 75:1231–1238. PMID: 8938756.
35. Zheng J, Lu Y, Perl ER. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J Physiol. 2010; 588:2065–2075. PMID: 20403977.

Fig. 1
DAMGO, a selective µ-opioid receptor agonist, produced outward currents in GABAergic interneurons of the SG.
(A) Identification of monosynaptic inputs from primary nociceptive C fibers to GABAergic interneurons. Superimposition traces of evoked EPSCs induced by monosynaptic C fiber inputs to GABAergic interneurons (conduction velocity (CV)=0.3 m/s). (B) In voltage clamp mode, DAMGO produced reproducible outward currents of about 39.55±2.16 pA in GABAergic interneurons. Holding potential (VH)=−70 mV.
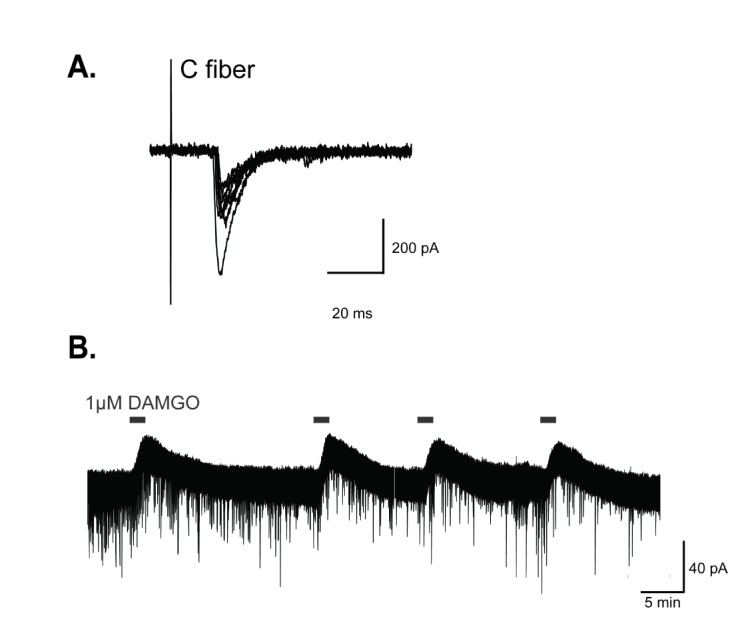
Fig. 2
DAMGO-induced current is mediated by K+ channels.
(A) Representative traces of current responses in the absence and presence of DAMGO in GABAergic interneurons. Voltage steps of 500 ms durations were commanded from −120 mV to −30 mV with 10 mV steps before and during superfusion with DAMGO. Holding potential (VH)=−70 mV. (B) The current-voltage (I-V) relationship of the DAMGO-induced current (n=6). (C) Schematic diagram of experimental protocol in (Ca) and representative traces of current responses of GABAergic interneurons (Cb) in the aCSF, DAMGO, and DAMGO added Ba2+. Voltage steps were commanded from −120 mV to −30 mV with 10 mV steps each lasting 500 ms. Holding potential (VH)=−70 mV. The I-V relationship shows that DAMGO-induced current is completely blocked by extracellular Ba2+ (Cc). (n=6, p<0.05, Two-way RM ANOVA).
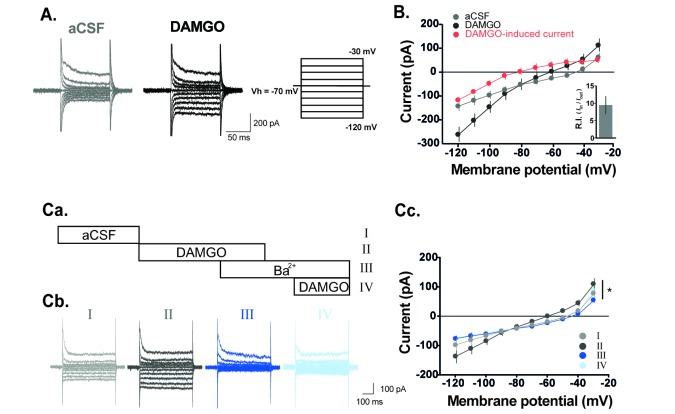
Fig. 3
DAMGO-induced current is dependent on external K+ concentrations.
(A) Representative traces of current responses at normal (2.5 mM) and high (8.5 mM) concentrations of the external K+ during DAMGO treatment. Voltage steps of 500 ms durations were commanded from −120 mV to −30 mV with 10 mV steps. (B) The DAMGO-induced current significantly increased at high K+ concentrations (n=5, p<0.05, Two-way RM ANOVA). Holding potential (VH)=−70 mV.
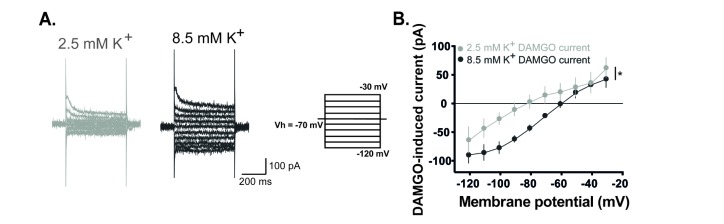
Fig. 4
DAMGO affects excitatory synaptic transmission between primary nociceptive C fibers and GABAergic interneurons in SG.
(A) Representative traces before, during, and after DAMGO treatment. (B) Evoked EPSCs were significantly decreased by DAMGO in GABAergic interneurons which receive monosynaptic nociceptive input from C fibers. The evoked EPSCs were decreased to 42.24±10.41% of baseline and recovered by 9.92±7.02 of baseline (n=12; p<0.01, paired t-test). Data are presented as mean±SEM.
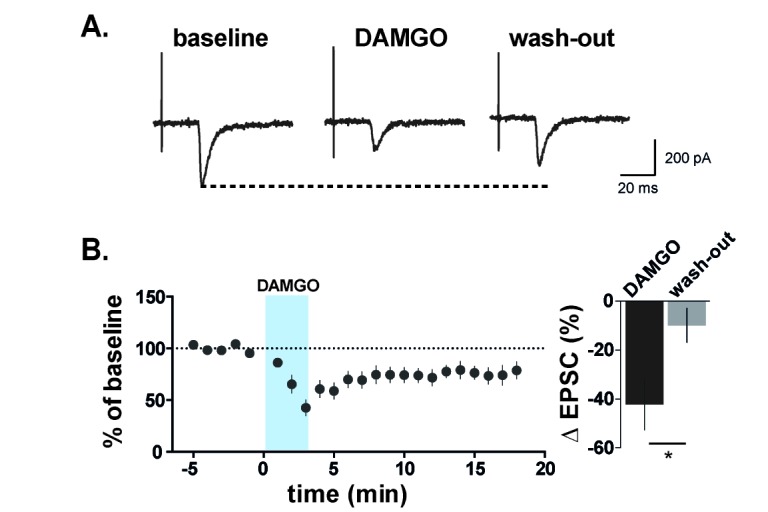
Fig. 5
A diagram to illustrate a possible mechanism by which DAMGO works in the SG.
(A) In spinal dorsal horn, excitatory neurons normally receive an inhibitory control of GABAergic interneurons, and as a result, pain transmission is properly modulated. In addition, these GABAergic interneurons inhibit each other. (B) Under DAMGO, the excitability of GABAergic interneurons which receive direct excitatory inputs from primary nociceptive C fibers is decreased. If so, the inhibition of another GABAergic interneurons (*) toward excitatory neurons can be enhanced. As a results, the sum of excitation of the entire spinal circuit will reduce the pain transmission.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download