Abstract
Sweet syndrome (acute, febrile, neutrophilic dermatosis) is characterized by the acute onset of an eruption of painful nodules or erythematous or violaceous plaques on the limbs, face and neck. These symptoms are accompanied by fever. The diagnostic features include histopathological findings of dermal neutrophilic infiltration without leukocytoclastic vasculitis or peripheral blood leukocytosis. Sweet syndrome is associated with infection, malignancies, autoimmune disease, pregnancy, and drugs. Patients with Sweet syndrome demonstrate a complete and rapid response to systemic steroid administration. Recently, a distinct variant of Sweet syndrome was reported, termed “histiocytoid Sweet syndrome”, in which the infiltration of myeloperoxidase-positive histiocytoid mononuclear cells are observed (in contrast to the infiltration of neutrophils). The other clinical features are similar to those of classic Sweet syndrome. Pediatric Sweet syndrome is uncommon, and the histiocytoid type is even rarer. To date, four cases of histiocytoid Sweet syndrome have been reported in children. Herein, we describe a case of histiocytoid Sweet syndrome in an otherwise healthy 10-year-old boy with no underlying systemic disease in whom non-steroidal, anti-inflammatory drug treatment was successful.
Sweet syndrome (acute, febrile, neutrophilic dermatosis) was first described in 1964 by Dr. Robert Sweet1. The clinical features of Sweet syndrome include the abrupt onset of painful, erythematous plaques or nodules on the limbs, face, and neck. These symptoms can be accompanied by fever or myalgia2. Histopathologically, Sweet syndrome is characterized by edema of the papillary dermis, fragmentation of neutrophil nuclei, and infiltration of mature neutrophils and lymphocytes within the dermis. Sweet syndrome has a worldwide distribution with no apparent racial predisposition23. Classical Sweet syndrome most commonly occurs in women between 30 and 50 years of age. However, Sweet syndrome has also been observed in younger adults and children34. The exact etiology is unknown, but the development of Sweet syndrome may be associated with several factors including viral infection of the upper respiratory tract, hematologic malignancies, autoimmune disease (dermatomyositis, lupus erythematosus, and relapsing polychondritis), inflammatory bowel disease (Crohn's disease and ulcerative colitis), pregnancy, and drugs (carbamazepine, celecoxib, and diazepam) 456.
Histiocytoid Sweet syndrome is a histopathological variant of Sweet syndrome6. The clinical features of histiocytoid Sweet syndrome are identical to the clinical features of classical Sweet syndrome67. However, histopathologically, histiocytoid Sweet syndrome is characterized by an infiltration of histiocytoid mononuclear cells rather than neutrophils8. The associated mononuclear cells exhibit immunoreactivity for CD15, CD43, CD45, CD68, mycobacterium avium complex (MAC)-386, human alveolar macrophage (HAM) 56, myeloperoxidase (MPO), and lysozyme suggesting that these histiocytoid-appearing, immature, myeloid cells are neutrophil precursors489.
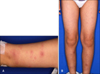
A 10-year-old boy was referred to the Department of Dermatology with erythematous macules and nodules on his extremities, which had been present for 9 days (Fig. 1). During the prior week, the patient experienced a fever of unknown origin (up to 38.4℃) and myalgia despite antibacterial therapy (intravenous nafcillin, ceftriaxone, clindamycin, and acetaminophen). With the exception of pneumonia one year prior, there were no related symptoms noted from the medical or family history. A physical examination revealed multiple, asymptomatic, erythematous macules and nodules on the extremities, which were not tender or itching. The lymph nodes and tonsils were not enlarged.
Laboratory tests demonstrated elevated neutrophils (80.3%), reduced lymphocytes (13.6%), and a normal white blood cell count (5,410/mm3). The tests also revealed elevated aspartate aminotransferase, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR), with values of 90 (normal: 7~38), 14.21 mg/dl (0~0.30) and 71 mm/h (1~15), respectively. The rheumatologic laboratory tests (antinuclear antibody and rheumatoid factor) and infectious disease diagnostic tests (Group A streptococcus, influenza, cytomegalovirus, mycoplasma pneumonia, quantiferon-TB, blood culture, respiratory syncytial virus polymerase chain reaction (PCR), throat swab and adeno virus Ag) were all negative. The cerebrospinal fluid (CSF) analysis (Cryptococcus Ag, gram stain, culture, Streptococcus pneumonia PCR, herpes simplex virus-PCR, enterovirus PCR and TB PCR) and urinalysis were negative. Chest X-ray and electrocardiography were normal. He didn't get any vaccination recently.
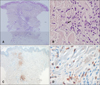
Histopathological examination of a skin biopsy obtained from a lesion on the right upper arm demonstrated infiltration of perivascular and perieccrine inflammatory cells in the mid- and deep-dermis (Fig. 2A). The infiltrating cells were large mononuclear cells with kidney-shaped nuclei, and the cells reacted positively with MPO. There was no evidence of true vasculitis (Fig. 2B~D).
Based on these findings, we diagnosed the patient with histiocytoid Sweet syndrome. Fever and the skin lesions were subsided spontaneously during treatment with non-steroidal, anti-inflammatory drug (ibuprofen, 40 mg/kg/day) after one week. There was no recurrence during the 10 months after treatment.
In this patient, the diagnosis of Sweet syndrome was based on the clinical features, laboratory findings, and histopathological features25. The diagnostic criteria for Sweet syndrome were initially established by Su and Liu6 and were modified by von den Driesch7. Two major and four minor diagnostic criteria are included. The major criteria are: 1) abrupt onset painful erythematous papules and nodules; and 2) histopathological evidence of neutrophilic infiltration without evidence of vasculitis. The minor criteria are: 1) fever over 38℃; 2) association with an underlying hematologic or visceral malignancy, pregnancy, or a preceding upper respiratory infection, gastrointestinal infection, or vaccination; 3) excellent response to treatment with systemic corticosteroid or potassium iodine; and 4) abnormal laboratory values upon presentation for at least three of the following four tests: ESR >20 mm/h, positive CRP, leukocyte >8,000, or neutrophils >70%. To establish a diagnosis of Sweet syndrome, the presence of both major criteria and two of the four minor criteria is required567.
Histopathologically, histiocytoid Sweet syndrome is characterized by edema in the papillary dermis and dense, inflammatory, cellular infiltration in the upper- to mid-dermis without vasculitis. These symptoms are also present with the classical Sweet syndrome25. In contrast, histiocytoid Sweet syndrome typically presents with an infiltrate containing predominantly mononuclear histiocytoid cells with large, slightly eccentric, kidney-shaped or elongated nuclei with eosinophilic cytoplasm810. The dermal infiltrating cells could be immature myeloid cells that are released from the bone marrow during the early stage of disease, and these cells may be replaced by mature neutrophils1112. Recently, Peroni et al.13 suggested that histiocytoid Sweet syndrome lesions are mostly infiltrated by M2-like macrophages and a few mature neutrophils. Classic Sweet syndrome presents with contrasting features. The histiocytoid cells positive stained with MPO, CD15, CD43, CD45, CD68, MAC-386, HAM56 and lysozyme. These markers correspond with the phenotype of immature neutrophils14.
The primary differential diagnoses of histiocytoid Sweet syndrome are leukemia cutis and Kikuchi-Fujimoto disease11516. Leukemia cutis resembles histiocytoid Sweet syndrome in its clinical and histopathological features; consequently, additional histopathological investigations, such as BCR/ABL FISH analysis, may be required to discriminate histiocytoid Sweet syndrome from leukemia cutis81516. Kikuchi-Fujimoto disease (histiocytic necrotizing lymphadenitis) is characterized by fever, rash, and localized lymphadenitis that is characterized by inflammatory cells accompanied by nuclear debris without granulocytes4. Although the definitive mechanism of histiocytoid Sweet syndrome pathogenesis remains unclear, recent studies have demonstrated that granulocyte colony-stimulating factor (G-CSF) might play an important role in its development. During classical Sweet syndrome, G-CSF levels in the peripheral blood are abnormally elevated in patients, and exogenous administration of G-CSF may induce the typical cutaneous lesions of Sweet syndrome12. However, in patients with histiocytoid Sweet syndrome, G-CSF levels in the peripheral blood remain low resulting in dysfunctional myeloid cell maturation1215.
Sweet syndrome is rare in children, and fewer than 80 pediatric cases have been reported in the literature to date. Sweet syndrome tends to present early in life (average age: 5 years). Both sexes are equally affected. However, a male predominance has been observed in children younger than 3 years of age. Sweet syndrome may be associated with the same predisposing conditions as the adult syndrome, including drugs, paraneoplastic disease, inflammatory disease, hematopoietic malignancies, or solid tumors. However, pediatric Sweet syndrome is more frequently associated with infectious diseases than the other factors1317.
Systemic corticosteroids are the treatment of choice for childhood patients with Sweet syndrome, although recurrence has been reported in ~20%~30% of patients when the corticosteroid dosage was tapered. However, the skin lesions generally resolve over a period of months without treatment101218.
Only four cases of childhood histiocytoid Sweet syndrome had been previously reported. Among these cases, two were associated with lupus erythematous, one had suspected Kawasaki disease, and the other had an upper respiratory infection, serositis, arthritis, and myocarditis1319. In contrast, the patient in our case study was healthy with no evidence of an underlying systemic disease. To the best of our knowledge, this is the first report of histiocytoid Sweet syndrome in a healthy child. Treatment with NSAIDs was successful.
Figures and Tables
References
1. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964; 76:349–356.
2. Cohen PR, Kurzrock R. Sweet's syndrome revisited: a review of disease concepts. Int J Dermatol. 2003; 42:761–778.

3. Jeanfils S, Joly P, Young P, Le Corvaisier-Pieto C, Thomine E, Lauret P. Indomethacin treatment of eighteen patients with Sweet's syndrome. J Am Acad Dermatol. 1997; 36:436–439.

4. Liu CI, Hsiao CH, Wu JT, Tsai TF. Sweet syndrome with histiocytoid infiltrate and neutropenia: a rare combination. J Am Acad Dermatol. 2009; 61:882–884.

5. Cooper PH, Innes DJ Jr, Greer KE. Acute febrile neutrophilic dermatosis (Sweet's syndrome) and myeloproliferative disorders. Cancer. 1983; 51:1518–1526.

6. Su WP, Liu HN. Diagnostic criteria for Sweet's syndrome. Cutis. 1986; 37:167–174.
7. von den Driesch P. Sweet's syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994; 31:535–556.

8. Chavan RN, Cappel MA, Ketterling RP, Wada DA, Rochet NM, Knudson R, et al. Histiocytoid Sweet syndrome may indicate leukemia cutis: a novel application of fluorescence in situ hybridization. J Am Acad Dermatol. 2014; 70:1021–1027.

9. Fernández-Torres RM, Castro S, Moreno A, Alvarez R, Fonseca E. Subcutaneous histiocytoid sweet syndrome associated with crohn disease in an adoloscent. Case Rep Dermatol Med. 2014; 2014:954254.
10. Uihlein LC, Brandling-Bennett HA, Lio PA, Liang MG. Sweet syndrome in children. Pediatr Dermatol. 2012; 29:38–44.

11. Srisuttiyakorn C, Reeve J, Reddy S, Imaeda S, Lazova R. Subcutaneous histiocytoid Sweet's syndrome in a patient with myelodysplastic syndrome and acute myeloblastic leukemia. J Cutan Pathol. 2014; 41:475–479.

12. Kawakami T, Ohashi S, Kawa Y, Takahama H, Ito M, Soma Y, et al. Elevated serum granulocyte colony-stimulating factor levels in patients with active phase of sweet syndrome and patients with active behcet disease: implication in neutrophil apoptosis dysfunction. Arch Dermatol. 2004; 140:570–574.
13. Peroni A, Colato C, Schena D, Rongioletti F, Girolomoni G. Histiocytoid Sweet syndrome is infiltrated predominantly by M2-like macrophages. J Am Acad Dermatol. 2015; 72:131–139.

14. Llamas-Velasco M, Concha-Garzón MJ, Fraga J, Aragüés M. Histiocytoid Sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol. 2015; 81:305–306.

15. Huang CF, Wu BY, Liaw FY, Wang WM, Chiang CP. Histiocytoid Sweet syndrome: report of two cases and review of the literature. Dermatologica Sinica. 2012; 30:71–74.

16. Anzalone CL, Cohen PR. Acute febrile neutrophilic dermatosis (Sweet's syndrome). Curr Opin Hematol. 2013; 20:26–35.

17. Hospach T, von den Driesch P, Dannecker GE. Acute febrile neutrophilic dermatosis (Sweet's syndrome) in childhood and adolescence: two new patients and review of the literature on associated diseases. Eur J Pediatr. 2009; 168:1–9.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download