Abstract
Background
VitabridC12 is newly developed and composed of vitamin C and Vitabrid (lamellar, hydrated zinc oxide).
Objective
In this study, we aimed to investigate the effects of VitabridC12 on psoriasis and atopic dermatitis.
Methods
Mice with imiquimod-induced psoriasis or Dermatophagoides farinae-induced atopic dermatitis were applied with VitabridC12. The effects of VitabridC12 were evaluated by clinical features, histology, and immunologic features by examining cytokines and chemokines.
Results
In psoriasis model, VitabridC12 decreased epidermal thickness and reduced inflammatory cell infiltration. In atopic dermatitis model, VitabridC12 decreased dermal infiltration of inflammatory cells, epidermal hyperplasia, and hyperkeratosis. VitabridC12 reduced the expression levels of proinflammatory mediators such as interleukin (IL)-1β, IL-6, IL-8, IL-17A, IL-22, tumor necrosis factor-α, CXCL1, CCL17, and CCL20 as well as COX-2 in imiquimod-induced psoriatic skin lesions. Likewise, VitabridC12 reduced the expression levels of IL-4, IL-5, IL-13, thymic stromal lymphopoietin, and CCL4 in D. farinae-induced skin lesions, and decreased the serum immunoglobulin E level in the atopic dermatitis mouse model. Particularly, the VitabridC12-treated mice showed downregulated expressions of mitogen-activated protein kinase (MAPK), including extracellular signal-regulated kinase (ERK), p38, and MAPK/ERK kinase, as well as inhibited phosphorylation of nuclear factor-κB p65.
Psoriasis and atopic dermatitis (AD) are chronic relapsing skin diseases characterized by dryness of the skin due to the impairment of the skin-barrier function1. Although the etiologies of both diseases have not been fully understood yet, studies on their pathogenesis have revealed that both are highly associated with the dysregulation of the immune system, mainly involving T cells. Psoriasis affects 2% to 3% of the whole population2. Its cause remains unknown even though genetic, environmental, and immunologic factors are known to be associated with the pathogenesis of psoriasis3. AD is characterized by severe pruritus and eczema. It has a prevalence of 10% to 20% in industrialized countries45. AD is highly associated with immune system imbalance, especially with T-helper (Th)2 immune responses6.
Vitamin C, also known as ascorbic acid, is a water-soluble ingredient found in fruits and vegetables. It has been traditionally utilized as an anti-oxidant and anti-aging agent7. However, vitamin C is unstable, easily oxidized, and decomposed into biologically inactive compounds8. In addition, intact vitamin C cannot easily penetrate the skin barrier due to its hydrophilic property. Therefore, a safer and stable delivery system for vitamin C needs to be developed by utilizing platforms such as microcapsules, nanospheres, or liposomes9. Choy et al.10 reported that unstable biological molecules can be successfully stabilized and effectively delivered to target tissues when they are encapsulated in a layered inorganic matrix of Vitabrid. Vitabrid is composed of the lamellar structure of hydrated zinc oxide (ZnO), which has been used in the management of many skin diseases because of its antioxidant, anti-inflammatory, and anti-bacterial effect11. Particularly, VitabridC12 is vitamin C incorporated into Vitabrid through the new technology of putting vitamin C in the layered complex of mineral carrier. It is composed of approximately 40-µm powdered particles. It formed into a nanoporous shell structure, which is coated with glyceryl monostearate. Glyceryl monostearate, emulsifier, can prevent the aggregation of vitamin C particles and increase skin's affinity values by hydrophobic modification of its surface.
The molecular mechanisms and therapeutic effect of VitabridC12, however, have not been clearly understood. In this study, we aimed to investigate the effect of VitabridC12 on skin diseases in psoriasis and AD mouse models by using imiquimod and Dermatophagoides farinae body (Dfb) extracts, respectively. Our results demonstrate that topical application of VitabridC12 significantly reduced skin lesions. The therapeutic action and effect of VitabridC12 on chronic inflammatory skin diseases was demonstrated in this study.
All animal care was performed in accordance with the Guide for the Care and Use of Laboratory Animals (Washington DC, USA) and was approved by the Animal Care and Use Committee of The Catholic University of Korea (2014-0090-01).
Female C57BL/6 and NC/Nga mice were purchased from Charles River Japan (Yokohama, Japan). These mice were all housed under conditions of controlled temperature (20℃~26℃), humidity (30%~70%), and lighting (lights on from 8 AM to 8 PM). Mice at 7 to 11 weeks of age were used for all the experiments and maintained in a specific pathogen-free barrier facility. VitabridC12 and Vitabrid were obtained from Hyundai IBT Ltd. (Seoul, Korea).
Mice at 8 weeks of age daily received topical application of 62.5 mg of commercially available imiquimod cream (5%) (Aldara; 3M Pharmaceuticals, St. Paul, MN, USA) for 6 consecutive days. Control mice were daily treated similarly with a control vehicle cream (Vaseline Lanette cream; Fagron, Rotterdam, The Netherlands) for 6 consecutive days. VitabridC12 or Vitabrid were daily applied into the mouse back, followed 12 hours later by topical treatment with imiquimod. We performed experiments with five mice in each group every experiment. We repeated this experiment three times. Four experimental groups were as follows: control vehicle group (Vaseline was applied twice a day), imiquimod group (imiquimod and Vaseline were applied daily with 12-hour interval), Vitabrid group (imiquimod and Vitabrid were applied daily with 12-hour interval), and VitabridC12 group (imiquimod and VitabridC12 were applied daily with 12-hour interval).
Dfb ointment, which is mixture of hydrophilic penetration and 5 mg of Dfb extract per gram hydrophilic petrolatum, was purchased from Biostir Inc. (Kobe, Japan). Hair on the upper back of the mice was removed with a clipper and a shaver 1 day before the experiment. Elicitation was performed by topical application of 100-mg Dfb ointment or ointment base (hydrophilic petrolatum) on the shaved dorsal skin and both surfaces of each ear. Barrier disruption was achieved by applying 150 µl of 4% sodium dodecyl sulfate on the shaved dorsal skin and both surfaces of each ear 3 hours before the Dfb ointment application. These procedures were repeated twice a week. We performed experiments with five mice in each group every experiment and repeated this experiment three times. Four experimental groups were as follows: control vehicle group (hydrophilic petrolatum was applied twice a week), Dfb group (Dfb ointment was applied twice a week), Vitabrid group (Dfb ointment was applied twice a week and Vitabrid was applied daily for 6 consecutive days. If Dfb ointment and Vitabrid needed to be applied on the same day, there was at least 12 hours' interval between Dfb ointment and Vitabrid treatments.), and Vitabrid C12 group (Dfb ointment was applied twice a week and Vitabrid C12 was applied daily for 6 consecutive days. Similar to Vitabrid, if Dfb ointment and VitabridC12 needed to be applied on the same day, there was at least 12 hours' interval between Dfb ointment and VitabridC12 treatments.).
Skin lesions taken from the imiquimod- and Dfb-treated mice were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 24 hours, washed with tap water, dehydrated with grade ethanol, and then embedded in paraffin. The paraffin blocks were cut in 4-µm-thick sections, mounted on glass slides, dewaxed, rehydrated with grade ethanol, and then stained with hematoxylin-eosin (H&E). Analysis was performed by using a microscope. All histological studies were performed in a blinded manner by two experienced dermatopathologists.
Total RNA was isolated from mouse skin by using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The first cDNA strand was synthesized from 1-µg total RNA by using a QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany). The QuantiTect primer assay (Qiagen) was used as primer sets for interleukin (IL)-1β, IL-4, IL-5, IL-6, IL-8, IL-13, IL-17A, IL-22, tumor necrosis factor (TNF)-α, CXCL1, CCL4, CCL17, CCL20, and thymic stromal lymphopoietin (TSLP). Glyceraldehyde-3-phosphate dehydrogenase messenger RNA was used as an endogenous control. Polymerase chain reaction (PCR) was performed by using Rotor-Gene 6000 (Corbett, Mortlake, Australia) and QuantiTect SYBR Green PCR Kit (Qiagen). The amplification program consisted of 1 cycle at 95℃ for 10 minutes, followed by 35 cycles at 95℃ for 20 seconds, 55℃ for 20 seconds, and 72℃ for 20 seconds.
Samples were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Pall Corp., Port Washington, NY, USA). Membranes were blocked for 1 hour in a blocking buffer (5% skim milk in 25 mM Tris-HCl [pH 8.0], 150 mM NaCl, and 0.1% Tween 20) and incubated overnight at 4℃ with specific primary antibodies for target signaling. Blots were washed and incubated with horseradish peroxidase-conjugated secondary antibodies for 2 hours at room temperature, and the signals were detected by using radiography after reaction with an enhanced chemiluminescence detection kit (iNtRON Biotechnology, Seongnam, Korea).
The amounts of total immunoglobulin E (IgE) in sera were determined by using sandwich enzyme-linked immunosorbent assay as previously described12. In brief, 96-well plates were coated with monoclonal anti-mouse IgE overnight at 4℃ and then further treated with 2% (w/v) bovine serum albumin dissolved in PBS containing 0.05% Tween 20 (PBS-T) for 2 hours at room temperature to block nonspecific binding. Next, serum samples were serially diluted in duplicated wells and incubated for 1 hour at room temperature. After 3 rinses with PBS-T, biotin-conjugated rat anti-mouse IgE Ab (LO-ME-2) was added to the wells, followed by the addition of streptavidin-peroxidase (Seikagaku Corp., Tokyo, Japan). After 3 rinses, the plates were developed by using a substrate solution containing 0.04% O-phenylenediamine dissolved in phosphate citrate buffer (pH 5.0). The reactions were terminated by the addition of sulfuric acid. The absorbance was read by using a microplate reader at 490 nm, and the amount of IgE isotype was calculated by comparison with the mouse IgE standard (Pharmingen, San Diego, CA, USA).
These results are representative of 3 independent experiments (n=5 for each group). Each data represents the mean value±standard error of three independent experiments. Statistical analyses were conducted with IBM SPSS Statistics ver. 19.0 for Windows (IBM Co., Armonk, NY, USA). Statistical analysis between multiple groups was performed using the one-way analysis of variance (ANOVA) (Kruskal-Wallis test) followed by Dunn's Multiple Comparison test. p-values <0.05 were considered statistically significant.
To examine the effects of VitabridC12 on psoriasis, psoriasiform dermatitis was induced on the back of the mice by the application of imiquimod daily for 6 days. Unlike the control group, the imiquimod group had induced skin swelling, which is the first hallmark of local skin inflammation (Fig. 1A). In addition, histopathological evaluation of the skin showed epidermal hyperplasia (acanthosis), hyperkeratosis, and increased dermal inflammatory cell infiltration in the imiquimod group compared to the control group (Fig. 1B). The thickness of the epidermis and dermis, and inflammatory cell infiltration significantly decreased in the Vitabrid or VitabridC12 group compared to the imiquimod group (Fig. 1C). Notably, the VitabridC12 group showed less inflammation than the Vitabrid group in the dermal thickness (p<0.05).
To examine the effects of Vitabrid C12 on the AD murine model, Dfb ointment was applied on the back of Nc/Nga mice twice for 3 weeks. Changes in the skin thickness after 3 weeks are shown in Fig. 2. Apparent acanthosis and edematous skin swelling occurred after the application of Dfb ointment in NC/Nga mice compared to the control group. Histological examination revealed an increased inflammatory response, including dense dermal infiltration of leukocytes, epidermal hyperplasia, and hyperkeratosis in the Dfb ointment-treated mice skin. As we expected, VitabridC12 was effective in decreasing these inflammatory responses (Fig. 2B). In the VitabridC12 group, the thicknesses of the epidermis markedly decreased, compared to the Dfb group (p<0.05, Fig. 2C). Interestingly, in toluidine blue staining, the mast cells more significantly decreased in the VitabridC12 group than in the Dfb control group (p<0.05, Fig. 3).
VitabridC12 was found to be effective in inhibiting the development of imiquimod-induced cutaneous inflammation in mice skin. Next, we performed quantitative real-time PCR analysis in mice skin to examine the mRNA expression levels of proinflammatory mediators. Similar to the histological changes, real-time PCR analysis revealed that the mRNA expression levels of proinflammatory cytokines such as IL-1β, IL-6, IL-8, TNF-α, IL-17A, and IL-22 increased in the imiquimod-treated mouse ears, whereas these mRNA expression levels decreased significantly in the VitabridC12 group (Fig. 4). VitabridC12 inhibited the mRNA expressions of IL-17A and IL-22, the signature cytokines of the Th17 cell lineage. Furthermore, treatment with VitabridC12 significantly hindered the mRNA expression levels of the other inflammatory chemokines such as CXCL1, CCL17, and CCL20, which are highly elevated in psoriasis131415.
We also investigated whether VitabridC12 downregulated the production of cytokines and chemokines in the Dfb-induced skin lesions of the NC/Nga mice. Quantitative real-time PCR analysis revealed that the mRNA expression levels of T cell-specific cytokines such as IL-4, IL-5, and IL-13 increased in the Dfb-treated mice ears, whereas VitabridC12 inhibited these mRNA expression levels. Compared to Dfb group, VitabridC12 is significantly inhibiting the mRNA expressions of TSLP, IL-4, IL-13 and CCL4 (p<0.05, Fig. 5A). Furthermore, VitabridC12 significantly downregulated the mRNA expression of TSLP, IL-4, and CCL4 more effectively than Vitabrid (p<0.05, Fig. 5A).
Then, we tried to investigate whether VitabridC12 could modulate the serum IgE levels in the Dfb-induced mice. The total IgE levels in the sera obtained from the mice are shown in Fig. 5B. The IgE levels increased in the Dfb-treated mice. The VitabridC12-treated mice displayed relatively lower levels of total serum IgE than Vitabrid-treated mice (p<0.05).
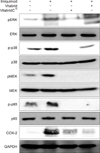
In addition, we examined whether VitabridC12 could inhibit mitogen-activated protein kinase (MAPK) signaling pathways that play key roles in inflammatory signaling in mammalian cells1617. Nuclear factor (NF)-κB p65 is the active subunit of NF-κB. Overactivation of NF-κB signaling leads to inflammatory disorders18. COX-2 is an inducible enzyme that converts arachidonic acid into prostaglandins and is involved in various inflammatory diseases19. VitabridC12 inhibited phosphorylation of MAPK/ERK kinase (MEK), extracellular signal-regulated kinase (ERK), and p38 in the imiquimod-treated mice. Moreover, VitabridC12 hindered phosphorylation of NF-κB p65 and reduced the expression levels of COX-2 in the imiquimod-treated mice (Fig. 6). Thus, we might speculate that VitabridC12 could downregulate cutaneous inflammation via inhibition of the MAPK kinase and NF-κB pathways.
VitabridC12 is a novel compound composed of a glyceryl monostearate -coated, layered vitamin C-Vitabrid complex. Vitamin C has long been regarded as a potent antioxidant agent20. However, because of its instability and the difficulty in delivering it into the dermis, many studies have been conducted to find stable compounds of and new delivery systems for vitamin C. Vitabrid is a hydrated ZnO in the lamellar structure, which causes minimal skin irritation10. ZnO also helps the skin restore disturbed skin-barrier function and enhance wound healing with its antimicrobial effects2122. It has also an anti-inflammatory effect by reducing neutrophil chemotaxis in psoriatic patients23. Because VitabridC12 is coated with hydrophobic glyceryl monostearate, the innovative substance penetrates into the skin more efficaciously than other vitamin compounds. Whereas vitamin C can be easily destroyed within 3 to 4 hours of exposure to air, VitabridC12 consistently releases vitamin C for longer than 12 hours. Furthermore, as VitabridC12 exchanges ions with perspiration (NaCl) from sweat glands and CO2 from the air, it can help in the absorption of vitamin C in the deep skin layer.
However, the biological and pharmacological activities of VitabridC12 are not well understood, specifically how it works in skin inflammatory diseases. Therefore, we investigated whether VitabridC12 could modulate skin inflammation in psoriasis and AD mouse models by using imiquimod and Dfb ointment, respectively. Topical application of VitabridC12 suppressed imiquimod-induced skin thickness and dermal inflammatory cell infiltration. The results suggested that treatment with VitabridC12 could exert significant anti-inflammatory effects on cutaneous inflammation. In addition, Vitabrid C12 significantly suppressed the expressions of proinflammatory mediators, including TNF-α, IL-1β, IL-6, IL-8, IL-17A, IL-22, CXCL1, CCL17, CCL20, and COX-2, which were elevated in psoriatic skin lesions2425262728293031.
Previous studies showed that in psoriatic skin lesions, the expression levels of ERK, JNK, and p38 were increased in the MAPK pathway163233. Therefore, we investigated the MAPK and NF-κB signaling pathways3435363738. Our study demonstrated that VitabridC12 could reduce the activities of p38, ERK, and MEK. While multiple signaling pathways, including NF-κB, are dysregulated in psoriatic epidermis39, VitabridC12 treatment reduced the phosphorylation of p65, a component of the NF-κB pathway. Our results showed that the therapeutic effect of VitabridC12 on the expression and production of proinflammatory mediators is associated with the inhibition of the MAPK kinase and NF-κB signaling pathways.
To study the effect of VitabridC12 on AD, we induced AD in NC/Nga mice by applying Dfb ointment. Our results showed that VitabridC12 efficiently suppressed the infiltration of inflammatory cells, including mast cells, and decreased the levels of the Th2 subset cytokines. Elevated serum IgE level is observed in most AD patients40. VitabridC12 also reduced the serum IgE level in the NC/Nga mice. AD keratinocytes secrete TSLP, which activates dendritic cells to prime naive T cells to produce IL-4 and IL-1341. In chronic AD, IL-5 is involved in eosinophil development and survival42. CCL4, also known as macrophage inflammatory protein 1β, is a CC chemokine with specificity for CCR5 receptors43. Serum CCL4 level is significantly elevated in patients with AD. Topical application of VitabridC12 significantly reduced AD symptoms and the levels of AD-related markers, including IL-4, IL-5, IL-13, CCL4, and TSLP. Sivaranjani et al.44 suggested that antioxidants such as Vitamin C may be helpful in the treatment of AD. We suggested that stable Vitamin C with Vitabrid may have synergic effect to AD.
The results of this study show that VitabridC12 reduce the cutaneous inflammation in psoriasis and AD model mice via modulating the complex network of cytokine-mediated signaling cascades. Moreover, VitabridC12 effectively inhibited diverse signaling pathways, including MAPK and NF-κB signaling. These results also propose that VitabridC12, a stable form of vitamin C with ZnO in the lamellar structure, has an enhanced ability to control skin inflammation. In conclusion, we propose the use of VitabridC12 as an effective treatment option for psoriasis and AD.
In summary, we observed that topical application of VitabridC12 decreased the number of inflammatory cells and the expression levels of diverse markers, thus reducing psoriasis or AD-like symptoms in the mice in this study. The effect of VitabridC12 might be associated with NF-κB and MAPK signaling pathways. Collectively, our results suggest that Vitabrid C12 is a promising candidate as a treatment option for management of psoriasis and AD.
Figures and Tables
Fig. 1
Effect of VitabridC12 on imiquimod-induced psoriasiform inflammation in BALB/c mice. (A) Skin lesions of psoriasiform inflammation. Images of skin lesions were taken in the 8th week. (B) H&E stained skin recovered in the 8th weeks (×100). VitabridC12 reduced acanthosis and inflammatory cell infiltration in the imiquimod skin lesions. (C) Epidermis thickness and dermal edema were evaluated at the 8th week. Vitabrid and VitabridC12 decreased skin thickness. Representative clinical presentations of the mice from the control group, imiquimod group, Vitabrid group, and VitabridC12 group are shown. These results are representative of 3 independent experiments (n=5 for each group). Each data represents the mean value±standard error of three independent experiments. *p<0.05.
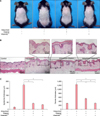
Fig. 2
VitabridC12 alleviated the Dermatophagoides farinae body (Dfb)-induced atopic dermatitis (AD)-like inflammation in the mouse model. (A) Skin lesions of AD-like skin inflammation. Images of skin lesions were taken in the 3rd week. (B) H&E stained skin recovered in the 3 weeks (×100). (C) Epidermal and dermal thickness were evaluated. VitabridC12 markedly decreased skin thickness; VitabridC12 reduced acanthosis and inflammatory cell infiltration in the AD-like skin lesions. Representative clinical presentations of the mice from the control group, Dfb group, Vitabrid group, and VitabridC12 group are shown. Data represent mean values±standard error (n=5). *p<0.05. The data shown are representative of three independent experiments.
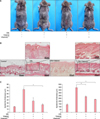
Fig. 3
The number of mast cells in the dorsal skin in Nc/Nga mice. (A) Histological assessment was performed by toluidine blue staining (×100). Purple colored cells were counted. (B) The number of mast cells in atopic dermatitis-like skin lesions and VitabridC12-treated skin lesions were determined. Purple cells (arrows) were counted. The number of cells at ×400 magnification was counted for each section using 3 randomly selected fields. Data represent mean values±standard error (n=5). *p<0.05. The data shown are representative of three independent experiments.
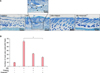
Fig. 4
VitabridC12 inhibited imiquimod-induced inflammatory responses in the mouse skin. Total RNA was isolated from the skin lesions of imiquimod-induced mice, and quantitative real-time polymerase chain reaction was performed to examine psoriasis-specific cytokines. VitabridC12 decreased the mRNA expression levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, IL-17A, IL-22, CXCL1, CCL20, and CCL17, and thereby ameliorated imiquimod-induced psoriasiform inflammation in the skin lesion. Data represent mean values±standard error (n=5). *p<0.05. The data shown are representative of three independent experiments.

Fig. 5
VitabridC12 inhibited Dermatophagoides farinae body (Dfb)-induced inflammatory responses in the Nc/Nga mouse skin. (A) Total RNA was isolated from the skin lesions of Dfb-treated mice, and quantitative real-time polymerase chain reaction was performed to examine atopic dermatitis-specific cytokines. VitabridC12 decreased the mRNA expression levels of thymic stromal lymphopoietin (TSLP), CCL4, interleukin (IL)-4, IL-5, and IL-13. Data represent mean values±standard error (SE) (n=5). *p<0.05. The data shown are representative of three independent experiments. (B) Immunoglobulin E (IgE) was quantified by performing an enzyme-linked immunosorbent assay. Representative clinical presentations of the mice from the control group, Dfb group, Vitabrid group, and VitabridC12 group are shown. Data represent mean values±SE (n=5). *p<0.05. The data shown are representative of three independent experiments.

Fig. 6
VitabridC12 inhibited cutaneous inflammation via the nuclear factor-κB signaling pathway. Protein extracts were prepared, and western blot analysis was performed with antibodies specific for the molecules indicated. pERK, p-p38, pMEK, p-p65, and COX-2 expression levels increased in psoriatic skin. The protein levels of pERK (44, 48 kDa), p-p38 (38 kDa), pMEK (44, 45 kDa), p-p65 (65 kDa), and COX-2 (75 kDa) decreased after treatment with VitabridC12. ERK: extracellular signal-regulated kinase, MEK: mitogen-activated protein kinase/ERK kinase, GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
References
1. Lee JH, Jung KE, Lee YB, Kim JE, Kim HS, Lee KH, et al. Use of emollients in atopic dermatitis: a questionnaire survey study. Ann Dermatol. 2014; 26:528–531.

2. Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin. 1996; 14:485–496.

4. Sampson HA. Atopic dermatitis. Ann Allergy. 1992; 69:469–479.
5. Leung DY. Atopic dermatitis: immunobiology and treatment with immune modulators. Clin Exp Immunol. 1997; 107:Suppl 1. 25–30.
6. Leung DY, Soter NA. Cellular and immunologic mechanisms in atopic dermatitis. J Am Acad Dermatol. 2001; 44:1 Suppl. S1–S12.

7. Blanck TJ, Peterkofsky B. The stimulation of collagen secretion by ascorbate as a result of increased proline hydroxylation in chick embryo fibroblasts. Arch Biochem Biophys. 1975; 171:259–267.

8. Yang JH, Lee SY, Han YS, Park KC, Choy JH. Efficient transdermal penetration and improved stability of L-ascorbic acid encapsulated in an inorganic nanocapsule. Bull Korean Chem Soc. 2003; 24:499–503.

9. Kaur IP, Kapila M, Agrawal R. Role of novel delivery systems in developing topical antioxidants as therapeutics to combat photoageing. Ageing Res Rev. 2007; 6:271–288.

10. Choy JH, Kwak SY, Park JS, Jeong YJ, Portier J. Intercalative nanohybrids of nucleoside monophosphates and DNA in layered metal hydroxide. J Am Chem Soc. 1999; 121:1399–1400.

12. Engelberts I, Möller A, Schoen GJ, van der Linden CJ, Buurman WA. Evaluation of measurement of human TNF in plasma by ELISA. Lymphokine Cytokine Res. 1991; 10:69–76.
13. Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009; 129:2175–2183.

14. Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ γδ T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol. 2011; 187:5026–5031.

15. Maeda S, Hayami Y, Naniwa T, Ueda R. The Th17/IL-23 axis and natural immunity in psoriatic arthritis. Int J Rheumatol. 2012; 2012:539683.

16. Johansen C, Kragballe K, Westergaard M, Henningsen J, Kristiansen K, Iversen L. The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br J Dermatol. 2005; 152:37–42.

17. Wang S, Uchi H, Hayashida S, Urabe K, Moroi Y, Furue M. Differential expression of phosphorylated extracellular signal-regulated kinase 1/2, phosphorylated p38 mitogenactivated protein kinase and nuclear factor-kappaB p105/p50 in chronic inflammatory skin diseases. J Dermatol. 2009; 36:534–540.

18. Sur I, Ulvmar M, Toftgård R. The two-faced NF-kappaB in the skin. Int Rev Immunol. 2008; 27:205–223.
19. Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998; 38:97–120.

20. Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992; 119:598–620.
21. Xhauflaire-Uhoda E, Henry F, Piérard-Franchimont C, Piérard GE. Electrometric assessment of the effect of a zinc oxide paste in diaper dermatitis. Int J Cosmet Sci. 2009; 31:369–374.

22. Lansdown AB, Mirastschijski U, Stubbs N, Scanlon E, Agren MS. Zinc in wound healing: theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007; 15:2–16.

23. Michaëlsson G, Ljunghall K. Patients with dermatitis herpetiformis, acne, psoriasis and Darier's disease have low epidermal zinc concentrations. Acta Derm Venereol. 1990; 70:304–308.
24. Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999; 113:752–759.

25. Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989; 86:6367–6371.

26. Degiulio R, Montemartini C, Mazzone A, Pasotti D, Donadini A, Ricevuti G. Increased levels of leukotriene B4 and interleukin-8 in psoriatic skin. Ann N Y Acad Sci. 1993; 685:614–617.
27. Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998; 111:645–649.

28. Fujita H, Shemer A, Suárez-Fariñas M, Johnson-Huang LM, Tintle S, Cardinale I, et al. Lesional dendritic cells in patients with chronic atopic dermatitis and psoriasis exhibit parallel ability to activate T-cell subsets. J Allergy Clin Immunol. 2011; 128:574–582.

29. Ono S, Otsuka A, Miyachi Y, Kabashima K. Severe psoriasis vulgaris exhibiting a high serum thymus and activationregulated chemokine level: possible association of T-helper 2 conditions. J Dermatol. 2013; 40:582–583.

30. Yalçin B, Tezel GG, Arda N, Erman M, Alli N. Vascular endothelial growth factor, vascular endothelial growth factor receptor-3 and cyclooxygenase-2 expression in psoriasis. Anal Quant Cytol Histol. 2007; 29:358–364.
31. Roh NK, Han SH, Youn HJ, Kim YR, Lee YW, Choe YB, et al. Tissue and serum inflammatory cytokine levels in Korean psoriasis patients: a comparison between plaque and guttate psoriasis. Ann Dermatol. 2015; 27:738–743.

32. Takahashi H, Ibe M, Nakamura S, Ishida-Yamamoto A, Hashimoto Y, Iizuka H. Extracellular regulated kinase and c-Jun N-terminal kinase are activated in psoriatic involved epidermis. J Dermatol Sci. 2002; 30:94–99.

33. Haase I, Hobbs RM, Romero MR, Broad S, Watt FM. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest. 2001; 108:527–536.

34. Faurschou A, Gniadecki R. TNF-alpha stimulates Akt by a distinct aPKC-dependent pathway in premalignant keratinocytes. Exp Dermatol. 2008; 17:992–997.

35. Lee CS, Lee SA, Kim YJ, Seo SJ, Lee MW. 3,4,5-Tricaffeoylquinic acid inhibits tumor necrosis factor-α-stimulated production of inflammatory mediators in keratinocytes via suppression of Akt- and NF-κB-pathways. Int Immunopharmacol. 2011; 11:1715–1723.

36. Pastore S, Mascia F, Mariotti F, Dattilo C, Mariani V, Girolomoni G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J Immunol. 2005; 174:5047–5056.

37. Sung YY, Kim YS, Kim HK. Illicium verum extract inhibits TNF-α- and IFN-γ-induced expression of chemokines and cytokines in human keratinocytes. J Ethnopharmacol. 2012; 144:182–189.

38. Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010; 1799:775–787.

39. Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009; 41:199–204.

40. Sampson HA, Albergo R. Comparison of results of skin tests, RAST, and double-blind, placebo-controlled food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 1984; 74:26–33.

41. Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010; 11:289–293.

42. Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994; 94:870–876.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download