Dear Editor:
The application of topical medications and moisturizers is the mainstay of treatment of atopic dermatitis1, but the complexity of the treatment and lack of self-care knowledge result in poor adherence, leading to therapeutic failure23. In order to improve the quality and reduce the cost of caring for patients with chronic diseases, various patient education programs have been applied and investigated4. Tailoring is the process of customizing health information to match select characteristics for each person5. In general, compared to non-tailored messages, tailored messages have better memory retention, and are read and perceived as more relevant6. However, there is a paucity of available information to assess the relative effectiveness of the different strategies, such as tailored education compared to non-tailored education, in atopic dermatitis. Thus, we conducted a pilot study to assess the potential of using tailored education in the field of dermatology, via assessment of moisturizer usage in patients with atopic dermatitis.
This prospective, randomized, controlled, pilot study consisted of a pre-education phase (week −1 to 0) and a post-education phase (week 0 to 1). From February 2015 through May 2015, 24 parents of children with mild-to-moderate atopic dermatitis were screened; 21 participants (parents of children with atopic dermatitis) were randomly assigned to either tailored or non-tailored education. The non-tailored education group received an educational leaflet and viewed a video portraying general recommendations for the use of moisturizers to manage patients with atopic dermatitis. The tailored education group received the same leaflet and video provided to the non-tailored group. In addition, the tailored group received an informative leaflet that described the body surface area (BSA) of individual children, the actual amount of moisturizer the participants had applied to their children, and the ideal amount of moisturizers based on the BSA of children. To eliminate the influence of different durations of education time, the tailored message in this study was very brief.
We provided commercial moisturizers to participants throughout the study period. The number of times moisturizer used, the eczema area and severity index (EASI) score, and the weight of the residual moisturizer were assessed throughout the study period. We defined the ideal application amount of moisturizer7 as 10 grams per BSA m2, and BSA was calculated from height and body weight using the DuBois method8. The study protocol was approved by the institutional review board of the SMG-SNU Boramae Medical Center (IRB no. 16-2014-154) and this study was registered at clinicaltrials.gov, NCT02343861. Written informed consent was obtained from all participants before enrollment.
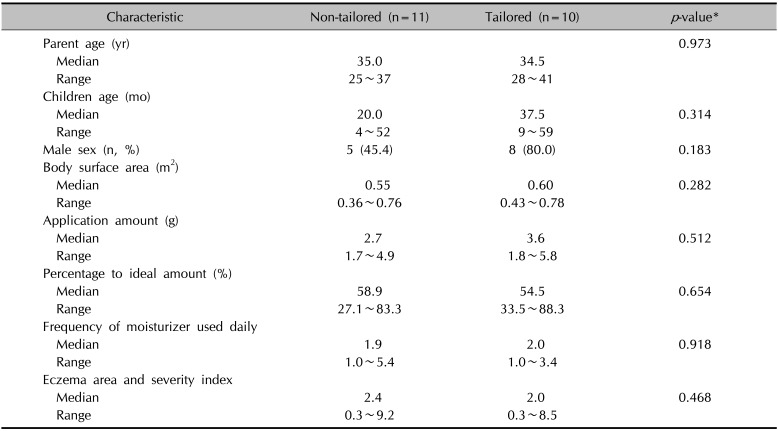
Twenty participants completed follow-up, and one participant from the tailored group was lost to follow-up. Demographics and baseline characteristics of participants are summarized in Table 1. The median BSA for children of all participants was 0.59 m2 (range, 0.36 to 0.78 m2; n=21). Before education, the overall median percentage to ideal application amounts was 56.6% (range, 27.1% to 88.3%; n=21). After receiving education, both groups showed significantly increased application amounts (p=0.007 for the non-tailored group; p=0.008 for the tailored group; by Wilcoxon signed rank test; Fig. 1); however, the application amount was significantly larger in the tailored group (median, 6.7 g; range, 4.0~8.1 g) than in the non-tailored group (median, 4.4 g; range, 1.9~7.4 g; p=0.031). Similarly, the percentage to ideal amounts was significantly larger in the tailored group (median, 99.0%; range, 92.6%~134.8%) than in the non-tailored group (median, 73.6%; range, 42.4%~135.1%; p=0.016).
In addition, all participants in the tailored group who completed follow-up achieved >90% of the ideal amount (n=9). In contrast, only 36% (n=4) of the participants in the non-tailored group achieved >90% of the ideal amount (p=0.005 by Fisher's exact test; Fig. 1); however, the number of moisturizer applied daily did not change after education. Although the EASI scores tended to be lower in the tailored group (median, 0.6; range, 0.3~3.7) vs. in the non-tailored group (median, 2.0; range, 0.3~9.2) after education, the difference was not statistically significant (p=0.095).
This study was designed as a pilot study with the small sample size, even though we found a significant difference in the amount of moisturizer used between the two groups. In addition, we only assessed the efficacy of education at 1-week follow-up; therefore, the long-term efficacy of tailored education remains unclear. In the present study, there were no significant differences in disease severity measured by EASI score between the two groups, even though disease severity score tended to be lower in the tailored group. This might be related to both the small sample size and the short-term follow-up.
In this pilot study, tailored messages improved the usage of moisturizer by parents of children with mild-to-moderate atopic dermatitis, compared to non-tailored education alone. Although non-tailored education significantly increased the amount of moisturizer used compared to the baseline level, the use of moisturizer increased significantly and consistently to a greater degree in the tailored group than in the non-tailored group. Further studies are needed to confirm whether tailored messages also have beneficial effects on the disease severity of atopic dermatitis and how the efficacy of education could change in longer follow-up periods.
References
1. Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014; 71:116–132. PMID: 24813302.
2. Krejci-Manwaring J, Tusa MG, Carroll C, Camacho F, Kaur M, Carr D, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007; 56:211–216. PMID: 17224366.
3. Lee JY, Her Y, Kim CW, Kim SS. Topical corticosteroid phobia among parents of children with atopic eczema in Korea. Ann Dermatol. 2015; 27:499–506. PMID: 26512163.

4. Weingarten SR, Henning JM, Badamgarav E, Knight K, Hasselblad V, Gano A Jr, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ. 2002; 325:925. PMID: 12399340.

5. Bull FC, Kreuter MW, Scharff DP. Effects of tailored, personalized and general health messages on physical activity. Patient Educ Couns. 1999; 36:181–192. PMID: 10223022.

6. Skinner CS, Campbell MK, Rimer BK, Curry S, Prochaska JO. How effective is tailored print communication? Ann Behav Med. 1999; 21:290–298. PMID: 10721435.

7. Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012; 26:1045–1060. PMID: 22805051.

8. Du Bois D, Du Bois EF. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916; XVII:863–871.
Fig. 1
Percentages to ideal amounts of moisturizer at pre- and post-education phases in participants who had children with mild-to-moderate atopic dermatitis (n=11 for the non-tailored group; n=10 for the tailored group). There is a missing line in the tailored group, because one participant from that group was lost to follow-up.
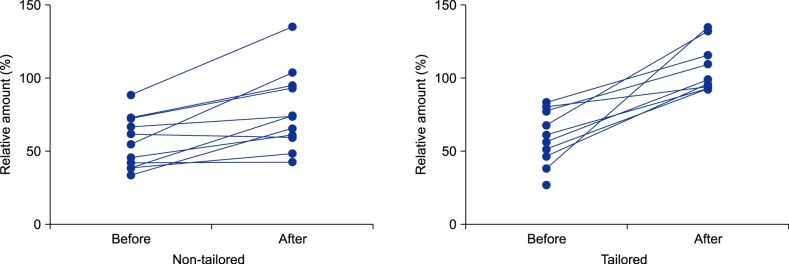
Table 1
Demographics, moisturizer use, and clinical characteristics before education




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download