Abstract
A 41-year-old female presented with complaint of left hip and buttock pain. Magnetic resonance imaging (MRI) showed multi-focal bone marrow signal intensity changes in left iliac bone, sacrum and femur with an area of necrosis. The primary radiological differential diagnosis was multi-focal tuberculous osteomyelitis. Subsequent pelvic bone biopsy and bone marrow biopsy confirmed the diagnosis of B-cell precursor acute lymphoblastic leukemia with extensive necrosis, which is infrequent in leukemia. When musculoskeletal symptoms precede peripheral blood abnormalities and MRI scanning reveals multi-focal necrotic lesions rather than diffuse signal change, it can be difficult to identify and/or advance leukemia as differential diagnosis.
Bone marrow involvement with leukemia usually manifests as a diffuse or seemingly normal anatomy on magnetic resonance imaging (MRI), and a focal finding of bone marrow involvement on MRI is rarely observed in patients with leukemia (1).
Musculoskeletal problems, including bone, joint, muscular pain, functional impairment and limping occurred in 20% to 40% of patients with acute leukemia as initial symptoms (2). Malignant osseous lesions often mimic infection or inflammation. These findings are not well visualized on MRI, or the findings are interpreted as something far more benign (i.e., a focal area of inflammation), and this can complicate the diagnostic picture. Therefore, any lesions thought to be suspicious for malignancy lesions must to be cultured and biopsied for more definitive tissue diagnosis.
Herein, we present a leukemia case with multi-focal bone marrow necrosis as diagnosed based upon the MRI evidence. The problem was initially thought to be osteomyelitis, but turned out to be B-cell precursor acute lymphoblastic leukemia (B-ALL), based upon on pelvic bone biopsy and bone marrow biopsy findings. This case report was approved by our Institutional Review Board and the requirement for the patient's informed consent was waived.
A 41-year-old female patient presented with complaint of left hip and buttock pain of two-months duration. The pain had recently been notably aggravated with exercise. She had fever of 38℃ at admission. The remaining medical history was unremarkable. Clinical examination peformed at the time revealed tenderness around the greater trochanteric area. There was no limitation in range of hip motion, however. The initial laboratory test revealed an elevated level of white blood cell count (12.13 × 103/µL), erythrocyte sedimentation (84 mm/hour), and C-reactive protein (58.16 mg/L). Based upon these findings, the initial clinical diagnoses included pyogenic arthritis of the left hip joint, and subtrochanteric fracture of left femur (because patient presented with left hip pain and fever).
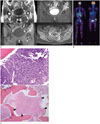
Plain radiographs of the cervical, thoracic, lumbar spine, pelvis and hip showed no discernible abnormal lesion. A subsequent hip MRI scan revealed multi-focal bone marrow signal changes in the left iliac bone, sacrum and the femurs, bilaterally (Fig. 1A). The lesions emited low signal intensity compared to the adjacent musculature andthe intervertebral disc on T1-weighted images, and heterogeneous intermediate to high signal intensity on T2-weighted images with rim enhancement on contrast enhanced T1-weighted images was appreciated, suggesting bone marrow necrosis. Additionally, the iliacus and gluteus musculature around the left iliac wing showed swelling and enhancement. Based on these image features focusing on the necrotic area and adjacent soft tissue involvement, the primary differential diagnosis was multi-focal osteomyelitis such as tuberculous osteomyelitis. Other differential diagnoses included chronic recurrent multifocal osteomyelitis (CRMO), Langerhans cell histiocytosis (LCH), hematologic malignancy such as lymphoma and metastases. A total body F-18-fluorodeoxy glucose positron emission tomography (PET) scan revealed evidence of intensely hypermetabolic lesions at the T10, T12 and L1 vertebral bodies, the bilateral sacral alae, left iliac wing and left femur (Fig. 1B). A subsequent whole spine MRI scan showed multi-focal uneven patchy abnormal signal intensity with subtle heterogeneous enhancement at T10, T12 and L1 vertebral bodies. Technetium-99m methylene diphosphonate bone scintigraphy was performed, which demonstrated accumulation only at the left ilium, and showing disparity with MRI and PET scan findings.
The patient received antibiotic treatment and underwent an incisional biopsy of the pelvic bone three days after admission. The pathologic diagnosis was B-ALL (Fig. 1C). On microscopic examination, the marrow space was found to be totally replaced by hyperchromatic blastic tumor cells, and the multi-focal area showed extensive ischemic necrosis. Immuno-histochemical staining was positive for CD10, TdT, and PAX5. A successive bone marrow biopsy was done and cytogenetic analysis revealed reciprocal translocation between one chromosome 9 and one chromosome 22, known as the “Philadelphia translocation.” The bone marrow blast count was 99%. Accordingly and not surprisingly, the white blood cell count rose to 31.78 × 103/µL and myelocytes and blasts formed cells were found in the peripheral blood. Cultures for bacteria and tuberculosis from the site of surgery were negative. The chemotherapy with hydroxyurea and imatinib mesylate started immediately, but the patient expired due to septic pneumonia only after five days.
A focal pattern of bone marrow involvement is rarely observed in patients with leukemia. It has been more often described in patients with leukemic relapse after treatment; in one study involving children with acute lymphocytic leukemia (ALL), a focal involvement instead of diffuse MRI pattern was observed in all eight patients with bone marrow relapse (13). Leukemia usually presents with a diffuse or a normal-appearing pattern of bone marrow findings on MRI scan (1). MRI findings, which relate to the condition of leukemia, generally include signs of bone marrow infiltration with decreased signal intensity on T1-weighted images, increased signal intensity on T2-weighted fat-saturated or short tau inversion recovery images and diffuse gadolinium enhancement. There is no literary consensus on staging leukemia with MRI using advanced techniques such as diffusion weighted image (DWI), quantitative MRI or qualitative MRI, although evaluation of bone marrow with DWI has been shown to be a relatively reliable predictor of the presence of leukemia even before a bone marrow biopsy is taken (4).
B-ALL and B lymphoblastic lymphoma (B-LBL) are classified as a spectrum disorder according to the 2008 WHO criteria. A diagnosis of B-ALL is the preferred diagnosis, over B-LBL, when there are more than 25% of lymphoblasts in the bone marrow (5). Although B-ALL with focal bone involvement is rare, there are a few case reports of B-LBL with primary bone involvement (67).
The main factor which seemingly facilitates the most common misdiagnosis is the necrotic nature of the lesions. Bone marrow necrosis may be associated with various infectious processes, malignancies (usually hematologic), medication use or underlying conditions such as sickle cell disease (8). The patient had fever and the inflammatory biomarkers were elevated. Therefore, multi-focal osteomyelitis such as tuberculous osteomyelitis had to be accepted as the working diagnosis in our case. However, the tuberculosis culture and polymerase chain reaction of biopsy specimens were negative. CRMO and LCH might serve to produce similar image findings, but tend to appear at a younger patient age. Although rare, malignant lesions such as lymphoma can mimic the signs and symptoms of, or arise from infection, and should be considered in differential diagnoses (7). Because she had no underlying malignancy, it was unlikely that the lesion would prove metastatic, but metastasis may also appear as rim-enhancing lesion (9).
Bone marrow necrosis is infrequent in acute leukemia. In one study of 640 patients with ALL and 1051 patients with acute myeloid leukemia, the incidence of bone marrow necrosis was 3.2% and 2.4%, respectively (10).
In our case, the microscopic examination of the pelvic bone biopsy revealed an extensive area of ischemic necrosis with preservation of the cortical bone (suggesting bone marrow necrosis). We thought bone marrow necrosis with peripheral enhancing rim as necrosis associated with osteomyelitis, but in fact it was necrosis arising from or related toa hematopoietic disorder. Bone marrow necrosis is known to radiographically manifest as extensive, diffuse, geographic patterns of signal abnormality consisting of a central area of variable signal intensity surrounded by a distinct peripheral enhancing rim (as seen on MRI scanning) (8). Badar et al. (10) suggested that bone marrow necrosis seen in acute leukemia is an infrequent occurrence, and suggestive of inferior response and poor prognosis. Our patient was also positive for “Philadelphia chromosome” on cytogenetic study, which is also generally accepted as a poor prognostic factor (5).
In summary, we described a case with multi-focal necrotic bone involvement documented by MRI scan. Based on the aggregate of the patient's complaints, the clinical findings and the radiographic evidence, we first suspected osteomyelitis and accordingly performed a biopsy. However, the biopsy result was (surprisingly) positive for B-ALL. As far as we know, there are only a few case reports of leukemia initially presented with multifocal necrotic bone marrow involvement, as in our case. There is significant overlap between the imaging findings which document infection and those which document malignancy.
Therefore, a biopsy should always be considered to rule out potential malignancy, in the absence of certain evidence of a benign condition.
Figures and Tables
Fig. 1
A 41-year-old woman with necrotic bone involvement on hip magnetic resonance imaging.
A. T1-weighted coronal image (left upper panel) shows a patchy area of low signal intensity at the left iliac wing (arrows). The T2-weighted coronal image (right upper panel) with fat suppression shows intermediate to high signal intensity with serpentine hyper-intense rim (arrows). Note that the adjacent gluteus musculature also shows high signal intensity (arrowhead). The gadolinium-based contrast enhanced T1-weighted coronal (left lower panel) and axial images (right lower panel) reveal evidence of rim enhancement at left iliac wing (arrows) and sacrum (arrowhead), thought to be most consistent with necrosis. The adjacent gluteus muscle also shows patchy enhancement.
B. A total body F-18-fluorodeoxy glucose positron emission tomography scan shows intense hypermetabolic lesions at T10, T12, L1 vertebral bodies, bilateral sacral alae and left iliac wing (arrows).
C. Photomicrograph of pelvic bone biopsy specimen (upper panel, H&E × 400) shows lymphoblastic cell infiltration, while the other area (under panel, H&E × 100) shows extensive necrosis (arrowheads). Note that bone trabeculae (arrows) are preserved, suggesting bone marrow necrosis.
H&E = hematoxylin and eosin stain
References
1. Moulopoulos LA, Koutoulidis V. MRI of the abnormal bone marrow: diffuse pattern. In : Moulopoulos LA, Koutoulidis V, editors. Bone Marrow MRI: a pattern-based approach. Milano: Springer;2014. p. 101–114.
2. Yoshikawa T, Tanizawa A, Suzuki K, Tanaka N, Hayashi T, Tsuda M, et al. The usefulness of T1-weighted magnetic resonance images for diagnosis of acute leukemia manifesting musculoskeletal symptoms prior to appearance of peripheral blood abnormalities. Case Rep Pediatr. 2016; 2016:2802596.

3. Kan JH, Hernanz-Schulman M, Frangoul HA, Connolly SA. MRI diagnosis of bone marrow relapse in children with ALL. Pediatr Radiol. 2008; 38:76–81.

4. Navarro SM, Matcuk GR, Patel DB, Skalski M, White EA, Tomasian A, et al. Musculoskeletal imaging findings of hematologic malignancies. Radiographics. 2017; 37:881–900.

5. Borowitz MJ, Chan JKC. B lymphoblastic leukemia/lymphoma with recurrent genetic abnormalities. In : Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Springer;2008. p. 171–175.
6. Kaygusuz I, Toptas T, Guven A, Firatli-Tuglular T, Tecimer T, Bayik M. Precursor B cell lymphoblastic lymphoma presenting as a solitary bone tumor: a case report and review of the literature. Int J Hematol. 2010; 92:757–761.

7. Mika J, Schleicher I, Gerlach U, Adler CP, Uhl M, Knoeller SM. Primary bone lymphomas thought to be osteomyelitis urgently demand a rapid diagnosis in bone pathology. Anticancer Res. 2012; 32:4905–4912.
8. Tang YM, Jeavons S, Stuckey S, Middleton H, Gill D. MRI features of bone marrow necrosis. AJR Am J Roentgenol. 2007; 188:509–514.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download