Abstract
Background
Rosacea is a chronic inflammatory disease characterized by centrofacial erythema. Excess cathelicidin is suggested to be important to the pathophysiology of the disease. Recently, presence of a vitamin D response element was revealed in the cathelicidin gene promoter.
Objective
The aim of this study was to determine whether vitamin D and cathelicidin are associated with rosacea, both serologically and histopathologically.
Methods
Subjects with rosacea and without chronic skin disorders were enrolled in the patient and control groups, respectively. Serum 25-hydroxy-vitamin D and cathelicidin levels were measured. Tissue expression of cathelicidin and vitamin D receptor were measured with immunostaining-intensity-distribution index.
Results
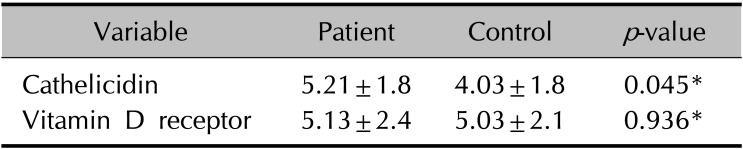
The mean serum 25-hydroxyvitamin D level of patients with rosacea was 12.18±5.65 ng/ml, which is lower than that of the controls (17.41±6.75 ng/ml). Mean serum cathelicidin levels in patients with rosacea and the controls were 85.0±26.1 ng/ml and 55.0±23.3 ng/ml, respectively. Cathelicidin expression in rosacea tissue was significantly higher than that in control tissue (5.21 vs. 4.03). No significant difference was observed in vitamin D receptor expression.
Conclusion
Higher cathelicidin expression in rosacea supports the hypothesis that an abnormal inflammatory response of the innate immune system is important in pathogenesis of rosacea, but the role of high cathelicidin serum levels is complicated. Serum vitamin D was lower in patients with rosacea, although serum cathelicidin was higher than that of the controls. This suggests that the role of vitamin D level in the pathogenesis of rosacea merits further investigation.
Rosacea is a disease characterized by erythema, telangiectasia, papules, and pustules on the face. It is a relatively common dermatologic disease that has a waxing and waning course. The precise pathogenesis of rosacea remains unknown, but it was recently reported that cathelicidin, an antimicrobial peptide (AMP) related to innate immunity, was increased in rosacea1. Cathelicidin is cleaved into the active peptide LL-37, which plays a role in cutaneous host defense. LL-37 is abnormally overexpressed in rosacea, and it has also been reported that the inflammatory response observed in rosacea did not appear when expression of the cathelicidin AMP gene in the mouse was inhibited1. Accordingly, the onset of rosacea may be related to increased expression of LL-37 and abnormalities in its metabolites.
A vitamin D response element was recently found in the promoter of the cathelicidin gene2. It is hypothesized that in a bacteria-infected lesion, active vitamin D3 is produced by activation of the inflammatory signal system and as a result, the expression of cathelicidin is increased3. Recent studies have shown that microorganisms such as Demodex, Staphylococcus epidermidis, and Bacillus oleronius may contribute to the pathophysiology of rosacea, and this might lead to the activation of vitamin D4. The purpose of this study was to reveal whether vitamin D and cathelicidin are associated with rosacea, both serologically and histopathologically.
The subjects of this study were patients between 18 and 69 years of age who had visited the Department of Dermatology, Hallym University Sacred Heart Hospital, from 2012 to 2013, and had been diagnosed with rosacea by a dermatologist. Subjects were clinically classified according to the U.S. National Rosacea Society Expert Committee (NRSEC)5. Subjects meeting or experiencing any of the following conditions were excluded: under medication for rosacea; concomitant chronic skin diseases; pregnancy or breast-feeding; cancer or central nervous system disease; patients who are receiving therapeutic intervention that might influence on the level of vitamin D level such as systemic corticosteroid, bisphosphonate, and vitamin D supplements.
For the comparison of the serum cathelicidin level, blood samples for the control group were obtained from patients between 18 and 69 years of age who had visited the same department during the same period but had not been diagnosed with rosacea or with any other chronic skin diseases by a dermatologist. Tissue samples for the control group were obtained from patients with a skin disease on the face that required surgical treatment, and the tissue samples were obtained from surplus normal tissues from specimen. Tissue samples from affected sites were biopsied for diagnosis and treatment. Tissue samples ranging from 2 to 2.5 mm in diameter and depth were collected from normal tissue around the affected sites. Exclusion criteria for the control groups were the same as those for the patient group. This study followed the guidelines of the Institutional Review Board of Hallym University Sacred Heart Hospital (IRB no. 2012-1009), and written consent was obtained from each participant.
The serum vitamin D level of the patient group was compared with the serum vitamin D level of the control group aged between 18 and 69 years who visited the hospital during the same period. Age and sex matched 34 subjects are selected as control group, who meet the same exclusion criteria for the patient group. Patient group were sampled mostly in fall and winter season. Serum samples from the controls obtained in approximately the same season were selected to minimize the differences for the seasonal changes in the level of vitamin D.
For the evaluation of vitamin D level, we measured serum 25-hydroxyvitamin D and 1 25-dihydroxyvitamin D using a competitive protein binding assay and radioreceptor assay, respectively. Serum cathelicidin was measured by enzyme-linked immunosorbent assay (ELISA) using an LL-37 ELISA kit (Hycult Biotech, Uden, The Netherlands). For serum cathelicidin, the average LL-37 level was compared based on the results of ELISA performed with the serum of the patient and control groups.
Immunohistochemical staining and western blotting was performed with the tissue sections of the patient and control groups to determine the expression of the vitamin D receptor. For immunohistochemical staining, paraffin microsections were cut, then stained using vitamin D receptor antibody (Novus Biologicals LLC, Littleton, CO, USA). Immunohistochemical staining and western blotting were performed for the same specimen, using cathelicidin antibody (Novus Biologicals LLC).
The degree of staining on immunohistochemistry was assessed using the immunostaining-intensity-distribution (IID) index6. The IID index was measured as follows: first, the epidermis was divided into 3 layers: basal, suprabasal, and superficial layers. For each layer, the intensity and stained cell proportion were assessed at a range of 0 to 3. The product of the 2 numbers was also calculated. The average of the 3 scores, respectively, for the three layers was taken as the final result. Expression of vitamin D and cathelicidin was also analyzed using the Zeiss LSM 700 confocal laser microscopy system (Carl Zeiss, Thornwood, NY, USA). Additionally, western blotting was performed for quantifying and comparing the protein expression in the tissues of the patient and control groups.
The correlation between the vitamin D or cathelicidin level and clinical severity was compared. The score for each item was obtained based on the standard grading system for rosacea established in 2004 by the NRSEC, and items showing a significant correlation were identified through multiple regression analysis.
For the patient group, 38 serum samples and 41 tissue samples were collected. For the control group, blood samples for the comparison of the vitamin D level were collected from 34 individuals, blood samples for the cathelicidin level were collected from 13, and tissue samples were collected from 11. Table 1 shows the demographics of each group. The mean ages of the patient groups for the serum and tissue samples were 48.7±7.7 years and 46.8±7.9 years, respectively. Those of the control groups for the vitamin D level and cathelicidin level were 49.7±8.1 years and 36.0±4.7 years, respectively. The mean age of the control group for the tissue samples was 32.1±18.0 years.
The clinical severity of 31 patients was assessed based on the standard grading system for rosacea established by the NRSEC (Table 2). When the patients were classified into clinical types according to major symptoms, 17 of the patients had disease of the papulopustular type and 12 had disease of the erythematotelangiectatic type (Table 2). No item showed a significant correlation with serum cathelicidin or vitamin D.
Table 3 shows the serum vitamin D levels of the patient and control groups. The average serum 25(OH) vitamin D in the patient group and control group was 12.18±5.65 ng/ml and 17.41±6.75 ng/ml, respectively. The difference was statistically significant (p=0.001). The average serum cathelicidin level in the patient group was 85.0±26.1 ng/ml, whereas the average serum cathelicidin level in the control group was 55.0±23.3 ng/ml. The difference was statistically significant (p=0.001). Pearson's partial correlation analysis was used to investigate the association between vitamin D level and cathelicidin level in patient group, and age and sex were controlled. The association was not statistically significant (partial correlation coefficient=−0.302, p=0.088).
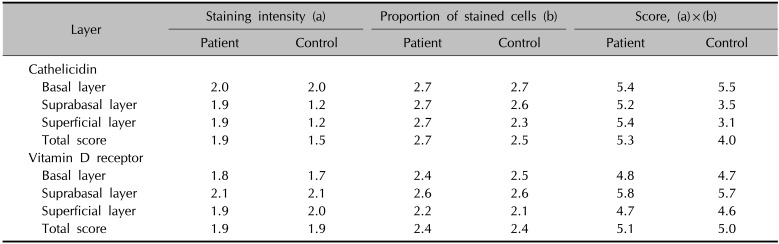
The degree of immunohistochemical staining for vitamin D receptor and cathelicidin in the patient and control groups was assessed using the IID index (Table 4). Vitamin D receptor antibody staining was mainly observed in the nuclei of keratinocytes, whereas cathelicidin staining was mainly seen in the cytoplasm of keratinocytes (Fig. 1, 2). The mean score on the IID index for vitamin D receptor was 5.13 in the patient group and 5.03 in the control group, and the difference was not statistically significant (Table 5). The mean score on the IID index for cathelicidin was 5.21 in the patient group and 4.03 in the control group, and this difference was statistically significant (Table 5). Western blotting was performed for 28 tissue samples of the patient group and 10 of the control group. However, the data did not show satisfactory results due to weak response, and no significant difference was observed in the expression of either vitamin D receptor or cathelicidin.
AMP is a molecule that plays an important role in the innate immunity of the skin surface and has been found to be an endogenous antibiotic. Like many other AMPs, CAMP encodes for a pro-peptide that is processed into the active form LL-37, and exists in proform as human 18-kDa cationic antimicrobial protein7. The function of LL-37 is to generate extensive antimicrobial activity by destroying the bacterial membrane and viral envelope8. It has also been proven with the use of animal experimental models that LL-37 has vasoactive and proinflammatory capacity910. LL-37 has been found to affect proinflammatory signaling such as toll-like receptor (TLR) signaling and epidermal growth factor receptor transactivation. Such affects are believed to play a role in the pathophysiology of various inflammatory skin diseases11. Rosacea is an inflammatory skin disease clinically characterized by repeated flushing, papules, and pustules. Currently, the precise pathophysiology of the disease has not been completely elucidated. It has recently been demonstrated that cathelicidin is increased in the lesions of patients with rosacea112. As in previous studies, the expression of LL-37 in the tissue of patients with rosacea was significantly higher than that in normal tissue in the present study. This finding confirms that an abnormal inflammatory response in the innate immunity is important in the onset of rosacea.
Serum cathelicidin is known to increase in infectious diseases13. The patients with rosacea in the current study showed significantly higher serum cathelicidin levels than those in the control group, although these patients are not thought to have any other conditions that may have influenced cathelicidin. Serum cathelicidin was increased in patients with rosacea as well as tissue cathelicidin expression in this study. However, the association between serum cathelicidin and tissue expression in pathogenesis of rosacea is unclear. Recently there have been inconsistent reports that the serum cathelicidin level is altered in other diseases such as psoriasis or atopic dermatitis1415. These findings suggest that the role of increased serum cathelicidin is complex, which requires further investigation. Various mechanisms have been suggested to explain the phenomenon of increased cathelicidin in rosacea. It is reported that cathelicidin-processing serine proteases such as kallikrein-5 (KLK5) were increased and the expression of TLR2 heightened in the skin of patients with rosacea1617. The increase of TLR2 may aggravate the susceptibility of the skin to external stimuli such as microbes and ultraviolet (UV) light. In addition, animal experiments have shown that TLR2 increases the production of KLK518. Recently, a vitamin D response element was found in the promoter of the cathelicidin gene2. In addition, it was found that activation of the inflammatory signal system passing through TLR2 augmented the action of 1α-hydroxylase (CYP27B1), an enzyme converting 25(OH)D3 into the activated form 1,25(OH)2D319. In consideration of the high recurrence rate in rosacea, the authors hypothesized that the systemic vitamin D level might be associated with the cathelicidin level in the skin and body. In this context, one study reveals that serum vitamin D levels are elevated in patients with rosacea compared to control group20. However, in the present study the vitamin D level in the patients with rosacea was lower than that in the controls, and the expression of vitamin D receptor in rosacea tissue was not significantly different from that in control tissue. Generally, vitamin D and cathelicidin are known to be positively correlated, in that CYP27B1 is expressed not only to activate vitamin D but also to induce cathelicidin21. However, in the present study the cathelicidin level rose despite decreased vitamin D. Rosacea is aggravated with sun exposure, so the patients might be more concerned with sun protection than the control group. That could be the possible reason for the low serum vitamin D level in this study. Another reason might be a bias from the small sample size. To elucidate this inconsistent status of vitamin D levels in patients with rosacea, larger epidemiological studies are required.
Recently, new mechanisms for the induction of CAMP other than the vitamin D receptor pathway have been suggested. It has been proven experimentally that cathelicidin may be increased via the pathway of nuclear factor-κB, CCAAT/enhancer-binding protein alpha by the stress of external stimuli on the endoplasmic reticulum22. In the present study, the absence of correlation between vitamin D and cathelicidin suggests the possibility that factors other than vitamin D may affect cathelicidin induction and inflammation in rosacea.
Limitations of this study include a potential selection bias such as the subjects were selected from a single tertiary hospital, and the study was conducted in an area with patients leading an urban lifestyle. Erythematotelangiectatic and papulopustular type were included together and different clinical severity together, that might cause bias in statistics. Whereas the control group for vitamin D level was matched for age and sex, the control groups for the serum cathelicidin and tissue samples differed in age and sex from the patient group because of the small number of the controls. The sample size was also small. As serum vitamin D level has individual variation, larger sample size is needed to evaluate vitamin D status. Individual differences in the level of UV exposure and skin phototype may also be confounding factors.
The present study is distinguished from previous studies in that, first, it revealed that the cathelicidin level is elevated significantly not only in tissues but also in sera of patients with rosacea. Rosacea recurs often during local maintenance therapies, and many cases respond favorably to systemic therapies such as oral antibiotics. These findings support the possibility that rosacea is a systemic inflammatory disease, which is according to recent reports, associated with insulin resistance and cardiovascular disease2324. Secondly, the current study demonstrated an increase in the cathelicidin level without an association of the vitamin D level. This suggests that the increased cathelicidin level in patients with rosacea might be induced by a vitamin D-independent pathway in addition to the vitamin D pathway. Further studies should clarify the manner in which such a relationship might contribute to the pathogenesis of rosacea.
ACKNOWLEDGMENT
This work was supported by a grant from Hallym University Medical Center Research Fund (01-2011-26).
References
1. Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007; 13:975–980. PMID: 17676051.

2. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005; 19:1067–1077. PMID: 15985530.
3. Schauber J, Gallo RL. The vitamin D pathway: a new target for control of the skin's immune response? Exp Dermatol. 2008; 17:633–639. PMID: 18573153.

4. Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015; 72:749–758. PMID: 25890455.
5. Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002; 46:584–587. PMID: 11907512.

6. Chaiyarit P, Kafrawy AH, Miles DA, Zunt SL, Van Dis ML, Gregory RL. Oral lichen planus: an immunohistochemical study of heat shock proteins (HSPs) and cytokeratins (CKs) and a unifying hypothesis of pathogenesis. J Oral Pathol Med. 1999; 28:210–215. PMID: 10226943.

7. Gennaro R, Zanetti M. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolymers. 2000; 55:31–49. PMID: 10931440.

8. Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006; 306:91–110. PMID: 16909919.

9. Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, et al. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005; 174:4271–4278. PMID: 15778390.

10. Koczulla R, von Degenfeld G, Kupatt C, Krötz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003; 111:1665–1672. PMID: 12782669.

11. Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006; 176:2455–2464. PMID: 16456005.

12. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013; 69(6 Suppl 1):S15–S26. PMID: 24229632.

13. Linde A, Lushington GH, Abello J, Melgarejo T. Clinical relevance of cathelicidin in infectious disease. J Clin Cell Immunol. 2013; S13:003.

14. Kanda N, Ishikawa T, Kamata M, Tada Y, Watanabe S. Increased serum leucine, leucine-37 levels in psoriasis: positive and negative feedback loops of leucine, leucine-37 and pro- or anti-inflammatory cytokines. Hum Immunol. 2010; 71:1161–1171. PMID: 20849904.

15. Kanda N, Watanabe S. Increased serum human β-defensin-2 levels in atopic dermatitis: relationship to IL-22 and oncostatin M. Immunobiology. 2012; 217:436–445. PMID: 22137028.

16. Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, Nakatsuji T, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011; 131:688–697. PMID: 21107351.

17. Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006; 20:2068–2080. PMID: 17012259.

18. Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011; 15:12–15.

19. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006; 311:1770–1773. PMID: 16497887.

20. Ekiz O, Balta I, Sen BB, Dikilitş MC, Ozuğuz P, Rifaioğlu EN. Vitamin D status in patients with rosacea. Cutan Ocul Toxicol. 2014; 33:60–62. PMID: 23713748.

21. Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008; 122:829–831. PMID: 19014773.

22. Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, et al. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011; 286:34121–34130. PMID: 21832078.

23. Duman N, Ersoy Evans S, Atakan N. Rosacea and cardiovascular risk factors: a case control study. J Eur Acad Dermatol Venereol. 2014; 28:1165–1169. PMID: 23909954.

24. Akin Belli A, Ozbas Gok S, Akbaba G, Etgu F, Dogan G. The relationship between rosacea and insulin resistance and metabolic syndrome. Eur J Dermatol. 2016; 26:260–264. PMID: 27328660.

Fig. 1
Immunohistochemical staining (vitamin D receptor; A, B: ×400) and confocal microscopy (vitamin D receptor; C, D: ×400) results for vitamin D receptor. Vitamin D receptor antibody was stained mainly in the nuclei of keratinocytes. On the immunohistochemical staining result, no significant difference was observed between patient (A) and control (B) groups. On the confocal microscopy, rosacea patient's specimen (C) showed slightly brighter enhancement at the epidermal nuclei than the control specimen (D).
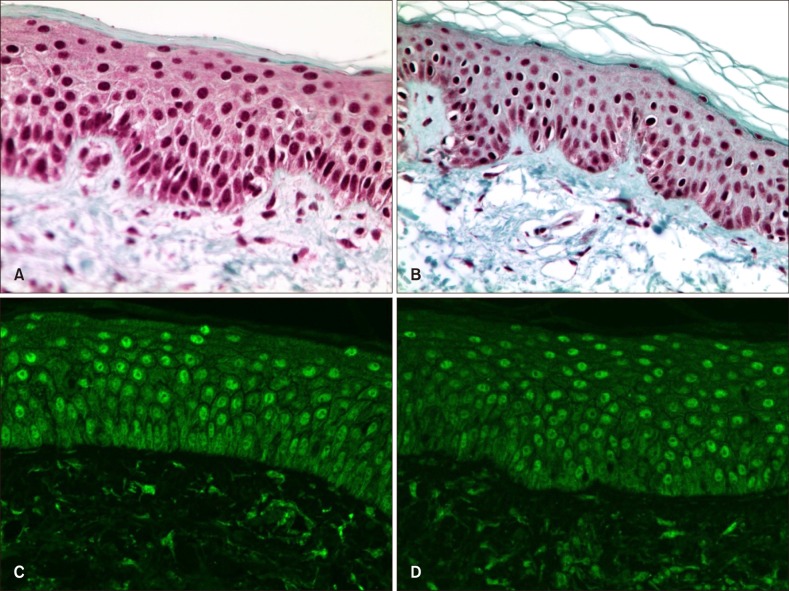
Fig. 2
Immunohistochemical staining (cathelicidin; A, B: ×400) and confocal microscopy (cathelicidin; C, D: ×400) results for cathelicidin. Cathelicidin antibody was stained mainly in the cytoplasm of keratinocytes. On the patient group (A) was stained more intensely than the control group (B). Also, on the confocal microscopy, rosacea patient's specimen (C) showed brighter enhancement at the epidermal cytoplasm than the control specimen (D).
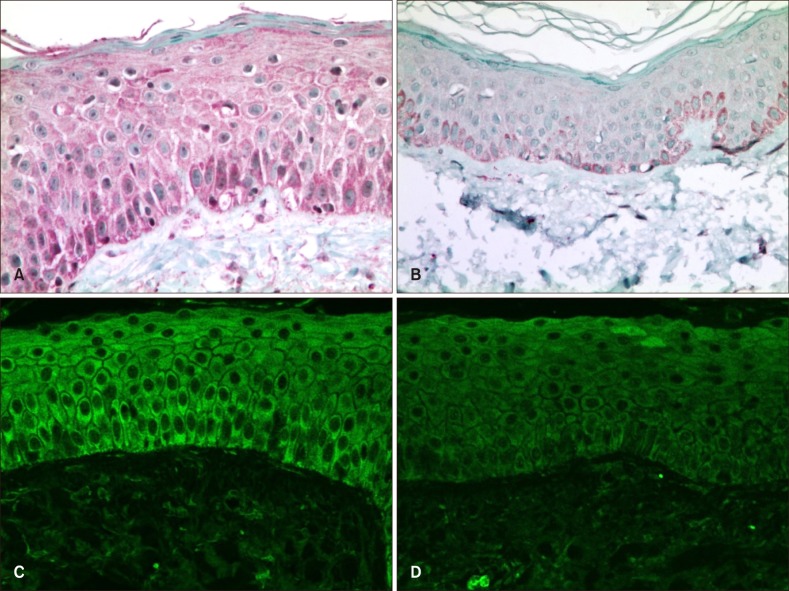
Table 1
Characteristics of the study groups
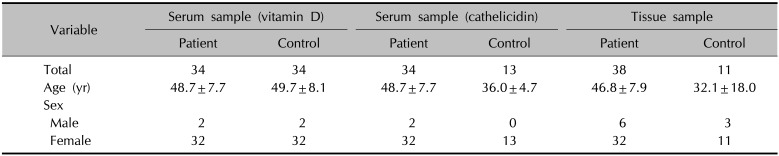
Table 2
Clinical features of rosacea patients (n=31)
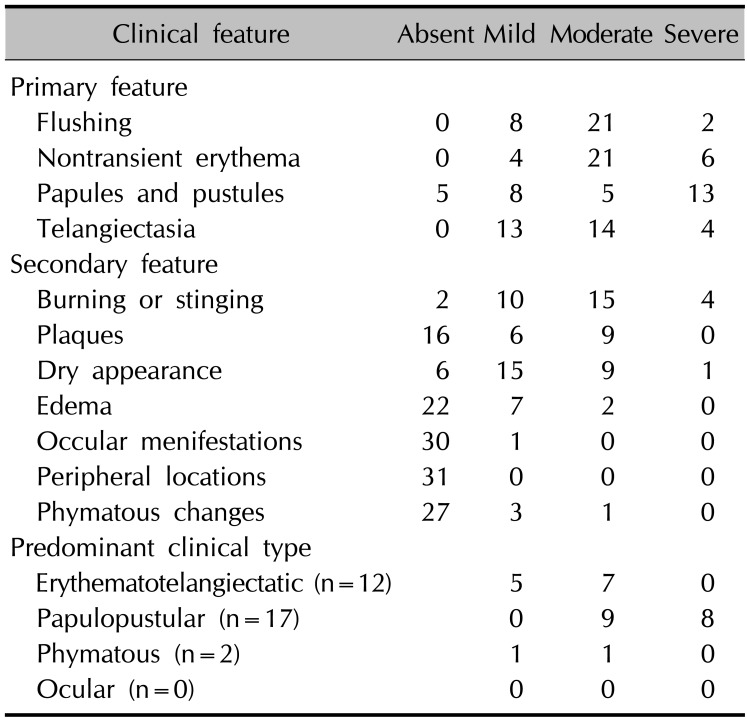
Table 3
Serum vitamin D and cathelicidin levels in the patient and control groups

| Variable | Patient (n=34) | Control | p-value | |
|---|---|---|---|---|
| Vitamin D (n=34) | Cathelicidin (n=13) | |||
| 25(OH) vitamin D (ng/ml)* | 12.18±5.65 | 17.41±6.75 | 0.001‡ | |
| Cathelicidin (ng/ml)† | 85.0±26.1 | 55.0±23.3 | 0.001‡ | |
Table 4
Average immunostaining-intensity-distribution index of tissue cathelicidin and vitamin D receptor




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download