Abstract
Purpose
Primary prophylaxis with granulocyte colony-stimulating factor can effectively prevent febrile neutropenia (FN) during breast cancer treatment. The aims of this study were to evaluate the incidence of FN and the ANC profile in patients undergoing chemotherapy and pegfilgrastim primary prophylaxis.
Methods
Patients receiving 6 cycles of adjuvant docetaxel, doxorubicin, and cyclophosphamide (TAC) chemotherapy were included in this study. Pegfilgrastim was administered with analgesics 24 hours after treatment. Laboratory tests were performed on day 0 (before chemotherapy) and ANC was measured daily starting day 5 until it were restored to 1,000/mm3. Bone pain was checked via the numeral rating scale (NRS).
Results
A total of 61 patients and 366 cycles were evaluated. Mean age was 49.2 ± 7.1 years. FN was seen in 5 patients (16.4%) and 12 cycles (3.3%) with pegfilgrastim. Grades 3 and 4 neutropenia was seen in 91.5% of cycles with FN. The ANC nadir was most commonly seen at day 7 and the mean ANC nadir depth was 265.7/m3. Age was negatively correlated with nadir depth (r = −0.137, P = 0.009). Severe pain higher than NRS 7 occurred in less than 20% of patients after the administration of pegfilgrastim.
Febrile neutropenia (FN) is one of the life-threatening adverse effects of breast cancer treatment. Performance status, nutritional status, advanced stage, prior episode of FN, and dose intensity are factors increasing the risk of FN. Prolonged duration of FN can interrupt chemotherapy treatment or lead to frequent hospitalization, thereby raising the burden of these patients. FN can also reduce relative dose intensity, which can impair the effect of chemotherapeutic treatment [1].
The use of granulocyte colony-stimulating factor (G-CSF) is helpful in decreasing incidence of FN [2]. Primary prophylaxis involves using G-CSF in the first and subsequent sessions of chemotherapy, whereas secondary prophylaxis involves the administration of G-CSF to patients who have experienced neutropenic complications following a treatment session. Treatment guidelines recommend to use G-CSF as primary prophylaxis during chemotherapy if the risk of FN is more than 20% [345]. Use of G-CSF is not considered to significantly increase the incidence of leukemia during chemotherapy for breast cancer [6].
Docetaxel-containing regimens have been used widely and adding docetaxel showed increased response to chemotherapy. However, these treatments have been associated with increased incidence of FN, 25.2% without G-CSF primary prophylaxis and 5.5% with primary prophylaxis [7]. Pegfilgrastim is a pegylated form of G-CSF that has longer half-life than short-acting G-CSF because of reduced renal clearance; therefore, it can be given once-per-cycle subcutaneously. In a study comparing G-CSF primary prophylaxis with pegfilgrastim (6 mg, once) versus daily administration of short-acting G-CSF (5 µg/kg/day for 5 days), FN incidence and related complications were significantly lower in the pegfilgrastim-treated group [8]. FN-related complications were lower with long-acting G-CSF treatment than with daily administration of short-acting G-CSF [910]. Adding prophylactic antibiotics was the most effective way to prevent FN during docetaxel, adriamycin, and cyclophosphamide (TAC) chemotherapy [8]. Although mild to moderate bone pain is frequently reported during G-CSF treatment [11], it can be managed by analgesics.
Adjuvant TAC chemotherapy is accompanied by high incidence of FN [12]. Primary prophylaxis with pegfilgrastim during adjuvant TAC and other high risk treatments is now covered by the Korean National Health Insurance System. In this study, we investigated the effect of G-CSF primary prophylaxis on ANC levels, FN incidence, toxicity of chemotherapy, and quality of life in breast cancer patients receiving TAC chemotherapy.
Female breast cancer patients who received adjuvant TAC chemotherapy were enrolled in this study. Doxorubicin (50 mg/m2), cyclophosphamide (500 mg/m2), and docetaxel (75 mg/m2) were given intravenously (day 1) every 21 days for 6 cycles. Pegfilgrastim (Neulasta, pegfilgrastim 6 mg), a pegylated G-CSF was administered subcutaneously between 24 hours to 48 hours after administration of chemotherapy (day 2). A nonsteroidal anti-inflammatory drug (naproxen, 500 mg, twice daily) was given for 5 days since day 2 immediately following pegfilgrastim treatment. Prophylactic antibiotics (ciprofloxacin, 500 mg, twice daily) and nystatin or chlorohexidine gargle were given in case of grade 4 neutropenia (ANC < 500/mm3), in accordance with Common Terminology Criteria for Adverse Events version 4.0 grading. Patient age at diagnosis, histology of breast cancer, stage, type of primary surgery, and menopausal status were recorded. Laboratory tests including complete blood test (CBC), liver enzyme, and creatinine levels were checked the day before chemotherapy. CBC was checked daily starting at day 5 until the ANC restored up to 1,000/mm3. For the ANC profile, we defined the lowest ANC level as ANC nadir, and if the nadir was not seen at day 7, we recorded the nadir day as −2 days, −1 day, or +1 day. FN was defined depending on whether the patient's temperature was >38.3℃ or remained ≥38.0℃ for over 1 hour with grade 4 neutropenia.
Severity of bone pain was determined by completion of a self-reporting questionnaire based on numeral rating scale (NRS) from days 1 to 5 in every cycle. Quality of life using the Functional Assessment of Cancer Therapy Questionnaire for Breast (FACT-B) questionnaire was evaluated at four time points: baseline, after 2nd cycle, after 6th cycle, and 4 months after the completion of chemotherapy. FACT-B total score is calculated by summation of physical well-being score, social/family well-being score, emotional well-being score, functional well-being score, and breast cancer subscale score. Scores of each subscale were assessed with reversal of negative items and considered missing items. This study was approved by Institutional Review Board of Soonchunhyang University Seoul Hospital (IRB No. 2017-11-011). Informed consent was obtained from all patients.
The correlation between body surface area (BSA), body weight, and age and ANC profiles from days 5 to 10 was assessed using Pearson correlation analysis (<0.5, weak correlation; 0.50–0.80, moderate correlation; 0.80–0.99, strong correlation). Statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
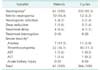
A total of 65 patients were enrolled. Data from 61 subjects and 366 cycles of chemotherapy were included in evaluation, with 4 patients excluded due to withdrawal of consent. The electronic medical records were reviewed. The mean age of patients was 49.2 ± 7.1 years. Mean weight was 59.7±1.0 kg and BSA was 1.6 ± 0.1m2. Most of the patients were more than T2 (71.1%). More than half of the patients (55.7%) were N1. The types of primary surgeries included breast conserving surgery (41.0%), mastectomy (36.1%), and skin sparing or nipple sparing mastectomy (23.0%). The clinical characteristics of the breast cancer patients are summarized in Table 1.
All patients experienced grade 4 neutropenia during at least one cycle. A few patients completed some sessions without experiencing severe (grade 3 or 4) neutropenia (31 cycles, 8.5%). Mean duration of severe neutropenia was 2.4 ± 1.6 days. Grade 4 neutropenia was seen in 83.3% of the cycles and mean duration was 1.8 ± 1.2 days. FN occurred in 16.4% of patients and 3.3% of all cycles throughout six sessions of chemotherapy. FN was most frequent in the first cycle (41.7%) followed by 5th cycle (33.3%). Mean duration of FN was 2.1 ± 1.4 days. All patients completed scheduled treatment without treatment interruption. One patient treated with reduced chemotherapeutic dose into 80% because of febrile neutropenia. Neutropenic infection was seen in five patients, which were anogenital herpes, pneumonia, urinary tract infection, and chemoport infection. Severe anemia was seen in 3.0% out of all cycles, with 8 cycles (2.2%) requiring blood transfusion. Except 1 patient who had significantly elevated AST and ALT, most patients did not present with severe hepatotoxicity and nephrotoxicity (Table 2).
The ANC profile is seen in Fig. 1. ANC decreased at day 6, and reached its lowest point at day 7. The mean ANC at day 7 was 375.3/m3 (0–4,860/m3) and the mean depth of ANC nadir was 265.7/m3 (0–4,760/m3). ANC nadir was seen at day 7 in most of the cycles (243 out of 366 cycles, 66.4%). Nadir at day 6 (−1 day) was seen in 29.2% of all cycles and nadir at day 8 (+1 day) was seen in 2.5%. The proportion of patients experiencing ANC nadir at −1 day increased as sessions continued (6.6% in cycle 1 and 39.3% in cycle 6). There was a negative correlation between ANC nadir and age, as determined by Pearson correlation analysis (r = −0.137, P = 0.009). There was no correlation between BSA or body weight with ANC nadir depth (r = 0.052, P = 0.328 and r = 0.042, P = 0.429, respectively). The duration and nadir depth showed moderate correlation (r = −0.586, P < 0.001). Age was also related to duration of neutropenia (r = −0.137, P = 0.009), and there were no relationships between duration and BSA or body weight.
Severe bone pain (>NRS 7) presented was shown at day 1 (chemotherapy) was 0.3 % of the patients and increased at day 2 (pegfilgrastim). Pain reached its highest level on day 5 (18.2%, Fig. 2). Quality of life measured by FACT-B decreased at midcycle, after chemotherapy, and four months after chemotherapy (Table 3). Physical well-being score decreased at midcycle (19.3 ± 7.5 vs. 16.8 ± 7.0), and did not recover to baseline levels until four months after chemotherapy (15.5 ± 8.7).
In this study, TAC chemotherapy with administration of long-acting G-CSF pegfilgrastim was useful towards completing the planned treatment without significant complications. Except for one individual, most of the patients received planned dose intensity. No life-threatening neutropenic infection occurred during the TAC chemotherapy.
The incidence of FN in this study was markedly lower compared to the incidence in Korean patients without pegfilgrastim-based primary prophylaxis (16.4% vs. 63.4%, respectively) [13]. We found the duration of grades 3 and 4 neutropenia lower (2.1 ± 1.4 days) than the previous report [14] (4.16 days) in Korean women. This was consistent with a previous report of TAC regimen, showing 17% of FN and 2% of grade 3/4 thrombocytopenia in TAC chemotherapy [15]. In a Japanese report, the incidence of neutropenia during TAC chemotherapy was 96.6%, and the incidence of FN was 3.4% of total cycles when using pegfilgrastim (6 mg), consistent with our study [16].
The musculoskeletal pain accompanying G-CSF administration is known to be related to bone marrow expansion, interaction with the nervous system, immune system modulation, or G-CSF's effect on bone metabolism [11]. However, pain is manageable with nonnarcotic analgesics [17]. Muscle pain and joint pain related to G-CSF (either short-acting or long-acting) presented most severely at days 3 to 6 in a previous report [18]. Our result showed more than 20% of patients had severe bone pain during days 6 to 8 (Fig. 2), suggesting that the pain could be related to the lowest ANC levels rather than pegfilgrastim injection.
In this study, we found significant correlation of old age with nadir depth and duration of neutropenia. Age is one of the characteristics of The Multinational Association for Supportive Care in Cancer (MASCC) risk index score [19] which is used in conjunction with other risk factors to predict low-risk FN patients [202122]. There has been a tendency to do dose capping to BSA 2 during chemotherapy in obese patients or applying ideal weight in overweight patients because of the concern of FN [23], bring about the lower incidence of FN. Since we did not modify dose intensity in obese or overweight patients, we did not find the correlation between body weight and duration of neutropenia or depth of ANC nadir. ANC nadir was found at day 7 in most of the cycles, consistent with previous reports [242526]. However, we found the proportion of ANC nadir on day 6 gradually increased as the sessions proceeded. Therefore, intensive patient education regarding adequate personal hygiene and food preparation should be required for the prevention of neutropenic infection.
No significant difference in 10-year disease free survival and overall survival was found when comparing concurrent and sequential administration of docetaxel (TAC 6 cycles every 3 weeks versus AC 4 cycles followed by docetaxel 4 cycles, respectively). The toxicity profile, however, is different in both arms, showing more incidence of myalgia and sensory neuropathy in AC-T arm [15]. From the results of Korean patients, TAC was associated with higher incidence of FN without primary prophylaxis, but showed similar quality of life compared to AC-T treatment [13]. In Korea, AC-T chemotherapy is only reimbursed during docetaxel sessions as secondary prophylaxis. Therefore, 6 cycles of TAC can provide shorter duration of treatment and could improve the quality of life during TAC with primary prophylaxis than without. If the patients are considered low risk from the MASCC risk index score, therapeutic strategies in an outpatient setting can be possible.
This study had some limitations. First, although it was a prospective study, we could not directly compare the effect of primary prophylaxis and secondary prophylaxis, the drug efficacy, and the side effects because it was conducted as a single arm observational study. Second, the sample size was relatively small so that the patient group of FN was limited when comparing the severity of bone pain and quality of life. Finally, we found that the FACT-B total score and all other items decreased during the treatment and did not recover to baseline 4 months after chemotherapy. However, several patient records at the 4-month time point were missing, so we could not make substantial conclusions regarding the recovery of quality of life.
In conclusion, the use of primary prophylaxis through long-acting G-CSF was associated with decreased incidence of FN in adjuvant TAC chemotherapy in breast cancer. Age was an important factor related to the duration of neutropenia and depth of ANC nadir. ANC nadir was mostly seen in days 6 and 7 of chemotherapy. Therefore, adequate supportive care and education for patient might be important during this period.
Figures and Tables
Fig. 1
ANC profiles after chemotherapy. (A) ANC shows lowest value at day 7 after chemotherapy. Panel B shows nadir depth of all cycles regardless of time after chemotherapy.

Fig. 2
Severe bone pain related to pegfilgrastim administration. Day 1 represents the day of chemotherapy. Severe pain more than numeral rating scale (NRS) 7 was shown less than 20% during days 3 to 5 after pegfilgrastim administration (day 2).

ACKNOWLEDGEMENTS
This study was supported by Kyowa Hakko Kirin Korea and also funded by the Soonchunhyang University Research Fund. This study was performed with the proper licensing agreement of Korean version of FACT-B.
Notes
References
1. Chirivella I, Bermejo B, Insa A, Perez-Fidalgo A, Magro A, Rosello S, et al. Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat. 2009; 114:479–484.

2. Clark OA, Lyman GH, Castro AA, Clark LG, Djulbegovic B. Colony-stimulating factors for chemotherapy-induced febrile neutropenia: a meta-analysis of randomized controlled trials. J Clin Oncol. 2005; 23:4198–4214.

3. Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011; 47:8–32.

4. Crawford J, Caserta C, Roila F. ESMO Guidelines Working Group. Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol. 2010; 21:Suppl 5. v248–v251.

5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines) [Internet]. Fort Wathington (PA): National Comprehensive Cancer Network;2018. cited 2018 Apr 16. Available from: http://www.nccn.org/professionals/physician_gls/f_ guidelines.asp.
6. Aapro M, Crawford J, Kamioner D. Prophylaxis of chemotherapy-induced febrile neutropenia with granulocyte colonystimulating factors: where are we now? Support Care Cancer. 2010; 18:529–541.

7. Martin M, Segui MA, Anton A, Ruiz A, Ramos M, Adrover E, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010; 363:2200–2210.
8. von Minckwitz G, Kummel S, du Bois A, Eiermann W, Eidtmann H, Gerber B, et al. Pegfilgrastim +/– ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol. 2008; 19:292–298.

9. Mitchell S, Li X, Woods M, Garcia J, Hebard-Massey K, Barron R, et al. Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: A systematic review. J Oncol Pharm Pract. 2016; 22:702–716.

10. Almenar D, Mayans J, Juan O, Bueno JM, Lopez JI, Frau A, et al. Pegfilgrastim and daily granulocyte colony-stimulating factor: patterns of use and neutropenia-related outcomes in cancer patients in Spain--results of the LEARN Study. Eur J Cancer Care (Engl). 2009; 18:280–286.
11. Lambertini M, Del Mastro L, Bellodi A, Pronzato P. The five “Ws” for bone pain due to the administration of granulocyte-colony stimulating factors (G-CSFs). Crit Rev Oncol Hematol. 2014; 89:112–128.

12. Cortes de Miguel S, Calleja-Hernandez MA, Menjon-Beltran S, Vallejo-Rodriguez I. Granulocyte colony-stimulating factors as prophylaxis against febrile neutropenia. Support Care Cancer. 2015; 23:547–559.

13. Lee J, Ahn MH, Jang YH, Lee EJ, Park JH, Rho J, et al. Toxicity and quality of life of Korean breast cancer patients treated with docetaxel-containing chemotherapy without primary G-CSF prophylaxis. Breast Cancer. 2014; 21:670–676.

14. Woo HD, Kim HS, Lee JH, Kim HM, Han SW, Kim SY, et al. Toxicity and tolerability study of adjuvant TAC regimen chemotherapy in Korean patients with breast cancer. J Breast Cancer. 2011; 14:Suppl 1. S44–S49.

15. Mackey JR, Pienkowski T, Crown J, Sadeghi S, Martin M, Chan A, et al. Long-term outcomes after adjuvant treatment of sequential versus combination docetaxel with doxorubicin and cyclophosphamide in node-positive breast cancer: BCIRG-005 randomized trial. Ann Oncol. 2016; 27:1041–1047.

16. Masuda N, Tokuda Y, Nakamura S, Shimazaki R, Ito Y, Tamura K. Dose response of pegfilgrastim in Japanese breast cancer patients receiving six cycles of docetaxel, doxorubicin, and cyclophosphamide therapy: a randomized controlled trial. Support Care Cancer. 2015; 23:2891–2898.

17. Kirshner JJ, Heckler CE, Janelsins MC, Dakhil SR, Hopkins JO, Coles C, et al. Prevention of pegfilgrastim-induced bone pain: a phase III double-blind placebo-controlled randomized clinical trial of the university of rochester cancer center clinical community oncology program research base. J Clin Oncol. 2012; 30:1974–1979.

18. Leung M, Florendo J, Kano J, Marr-Del Monte T, Higgins B, Myers R, et al. A modified filgrastim regimen does not reduce pain burden compared to pegfilgrastim in women receiving chemotherapy for non-metastatic breast cancer. Support Care Cancer. 2015; 23:1669–1677.

19. Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, et al. The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000; 18:3038–3051.

20. Lyman GH, Kuderer NM, Crawford J, Wolff DA, Culakova E, Poniewierski MS, et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer. 2011; 117:1917–1927.

21. Ahn S, Lee YS, Lee JL, Lim KS, Yoon SC. A new prognostic model for chemotherapy-induced febrile neutropenia. Int J Clin Oncol. 2016; 21:46–52.

22. Bozcuk H, Yıldız M, Artac M, Kocer M, Kaya C, Ulukal E, et al. A prospectively validated nomogram for predicting the risk of chemotherapy-induced febrile neutropenia: a multicenter study. Support Care Cancer. 2015; 23:1759–1767.

23. Lote H, Sharp A, Redana S, Papadimitraki E, Capelan M, Ring A. Febrile neutropenia rates according to body mass index and dose capping in women receiving chemotherapy for early breast cancer. Clin Oncol (R Coll Radiol). 2016; 28:597–603.

24. Lee KH, Kim JY, Lee MH, Han HS, Lim JH, Park KU, et al. A randomized, multicenter, phase II/III study to determine the optimal dose and to evaluate the efficacy and safety of pegteograstim (GCPGC) on chemotherapy-induced neutropenia compared to pegfilgrastim in breast cancer patients: KCSG PC10-09. Support Care Cancer. 2016; 24:1709–1717.

25. Green MD, Koelbl H, Baselga J, Galid A, Guillem V, Gascon P, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003; 14:29–35.
26. Holmes FA, O'Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002; 20:727–731.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download