Abstract
Limb-girdle muscular dystrophies (LGMD) are heterogeneous disorders with autosomal inheritance. Autosomal dominant LGMD mapped to 7q36.3 has been classified as LGMD type 1D (LGMD1D) in the Human Gene Nomenclature Committee Database. LGMD1D is characterized predominantly by limb-girdle weakness and may also show a bulbar symptom in some cases. In the past, the frequency of this disease was uncommon, and this disorder was mainly found in Europe and the United States. However, recently, this disorder has been reported in Asia, including Japan, Korea, and Taiwan. Here, we report on three LGMD1D patients, including one with a novel mutation in DNAJB6, c.298T>A. While two patients complained of limb-girdle weakness, as would be expected, one patient had distal weakness. They had various serum creatine kinase levels. Radiologic findings in one patient showed fatty degeneration and atrophy in the posterior part of distal muscles. Pathologic findings in one of the patients showed rimmed vacuoles. Although LGMD1D is still uncommon in Korea, we discovered three Korean patients with LGMD1D, including one novel mutation in DNAJB6, p.Phe100Ile (c.298T>A).
Limb-girdle muscular dystrophies (LGMD) are hereditary progressive myopathy disorders presenting with predominant weakness of axial and proximal muscles. Among them, LGMD type 1D (LGMD1D) is a disease caused by mutation in the DNAJB6 gene.1 LGMD1D is characterized by an onset in adults and slow progression, although a few patients do have symptoms in their childhood. Limb-girdle muscle weakness with waddling gait is common, and most patients have slightly elevated serum creatine kinase (CK) levels. Muscle biopsy typically shows rimmed vacuoles in skeletal muscle.2 Here, we describe three patients with LGMD1D from two families. One patient was found to have LGMD1D mutation (p.Phe89Ile) by whole axon sequencing analysis, while the other two patients were found to have p.Phe100Ile missense mutation by targeted sequencing.
This study has been approved by the Institutional Review Board (IRB) of Gangnam Severance Hospital (IRB no. 2015-0529-004).
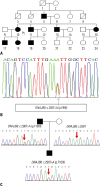
A 56-year-old woman (patient 1) visited the neurology outpatient clinic due to lower extremity weakness. She had experienced minimal weakness in limb-girdle muscles during her 4th decade, and these symptoms had slowly deteriorated over 20 years. Neurological examination revealed bilateral symmetric weakness of limb-girdle muscles. The patient's limb-girdle muscle strength was grade 4 under the Medical Research Council (MRC) system, and her distal muscle strength was almost normal. She could also walk independently with no symptoms of dysarthria, dysphagia, or dyspnea. Electrocardiogram showed normal sinus rhythm, and she had no cardiac symptoms. Laboratory findings showed normal serum CK levels (175 U/L) and electromyography showed myopathic changes. On leg magnetic resonance imaging, moderate fatty change was noted in the tibialis posterior and gastrocnemius (Fig. 1A). Her father (Fig. 2A) had suffered from the same symptoms, and her son also had similar symptoms. Whole exon sequencing was performed and revealed a heterozygous missense mutation (c.265T>A, p.Phe89Ile) in DNAJB6 (Fig. 2B).
A 31-year-old man (patient 2) visited a neurologist because of bilateral lower extremity weakness. He reported not running well since childhood, although he lived without any problems in his daily life. For a year before visiting the hospital, he had difficulty climbing stairs and needed a handrail when climbing them. On physical examination, bilateral atrophy of the thigh muscles was seen, and toe gait or heel gait seemed to pose difficulty. He had no signs of dysarthria or dysphagia or any cardiac symptoms. Laboratory tests showed mild elevation of CK (613 U/L), and myopathic findings were found on electromyography.
The father of patient 2, a 63-year-old man (patient 3), suffered from leg weakness since he was 17 years of age. When he visited our hospital at the age of 37 years, he showed prominent lower extremity weakness and mild weakness of the upper extremity. Ten years later he was unable to walk, and dysarthria and dysphagia had emerged. The patient's distal lower extremity muscle strengths were MRC grade 1, whereas his hip muscle strengths were rated between MRC grades 2 and 3. Likewise, distal muscles in upper extremities showed more pronounced weakness than proximal muscles. Electromyography showed brief short motor unit action potentials in multiple muscles. His muscle pathologic study showed myopathic features with rimmed vacuoles (Fig. 1B).
At the age of 63, he and his son (patient 2) visited our clinic and were confirmed for a novel missense mutation in DNAJB6 gene, c.298T>A (p.Phe100Ile) by a targeted next generation sequencing with 194 genes associated with muscular dystrophies (Fig. 2C).
LGMD1D is linked to chromosome 7q36.3 with a change in exon 5 of DNAJB6. It is characterized by progressive proximal muscle weakness, even though one family showed distal dominant weakness.2 Some reported studies have shown similar symptoms regardless of ethnicity or genetic variants. In muscle pathology, myopathic changes and rimmed vacuoles can be found. According to previous studies, the general estimated incidence of LGMDs range from 1 to 6 out of 100000.1 However, because of the heterogeneity of LGMDs, their prevalence is underestimated, and the frequency is likely to increase if more genetic and pathologic information accumulates.
Our patients experienced gradually progressive weakness with various serum CK levels. They all had progressive limbgirdle muscle weakness, and patient 3 also had bulbar symptoms. We discovered heterozygous missense mutation of the DNAJB6 gene on whole axon sequencing and target sequencing panel, including 191 genes associated with muscular dystrophies (c.265T>A for patient 1 and c.298T>A for patients 2 and 3).
Unfortunately, we were unable to obtain genetic information from the family of patient 1. However, her father, son, all of her siblings, and one nephew had progressive weakness throughout their lifetime. This pedigree suggests that the disease is transmitted in an autosomal dominant inheritance pattern.
Although patients with LGMD1D are predominantly characterized by proximal muscle weakness, patient 3 had distal dominant weakness and bulbar symptoms. Ruggieri, et al.3 reviewed the cases of five patients and conducted a formal study with LGMD1D patients. They found that proximal G/F domain mutations (Phe89, Phe91, and Phe93) caused proximal limb-girdle weakness, while distal G/F mutations (Pro96 and Phe100) caused distal-onset weakness. Our patient also had distal G/F mutations (Phe100).
In previous studies, p.Phe89Ile45 was identified as a pathogenic mutation, and mutation of the 298th base pair in exon 5 was reported in one case.3 Our patient, however, did not have the same missense mutation. One study reported a mutation of c.298T>G, and our study discovered a mutation of c.298T>A. The proteins synthesized also differed in valine and isoleucine residues. According to the criteria of American College of Medical Genetics and Genomics, a novel missense amino acid change occurring at the same position as another pathogenic missense change is considered moderate evidence for being pathogenic.6 Also, this variant was not shown in controls in population frequency studies from the Exome Sequencing Project, 1000 Genomes Project, or Exome Aggregation Consortium. Furthermore, mutations in the G/F domain have been indicated as possible mutations in previous studies. Based on this evidence for moderate pathogenicity, we concluded that this variant is likely pathogenic.
More than nine mutation sites in the DNAJB6 gene have been identified, including those in our study. Some of them, including p.Phe91Ile, p.Phe93Leu, p.Pro96Arg, and p.Phe100Val, have been reported in European and American families.23457 Some of them, such as p.Phe93Ile and p.Pro96Leu, have only been reported in Asian families.8910 The mutations of p.Phe89Ile and p.Phe91Leu were reported in European, American, and Asian families. One of our cases had a mutation of p.Phe100Ile, which is a novel mutation that has not been reported before. The symptoms of patients do not appear to differ significantly between Eastern and Western countries.
The diagnosis of muscular dystrophy is difficult, and it is not easy to find the pathologic mutation. In addition to clinical symptoms, family history and serum CK levels should be considered. Continued reporting of clinical symptoms and genetic variation will be helpful in diagnosing these conditions. Here, we report three patients with LGMD1D from two families and one novel mutation in DNAJB6, p.Phe100Ile (c.298T>A).
Figures and Tables
Fig. 1
Radiologic and pathologic findings of muscular dystrophy patients. (A) Radiologic finding of patient 1. T2-weighted MRI shows moderate fatty change in the tibialis posterior (arrowhead) and gastrocnemius (arrow). (B) Muscle biopsy in patient 3 shows rimmed vacuole findings (arrow) (hematoxylin and eosin stain, ×200), scale bar, 50 µm.
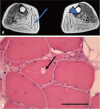
Fig. 2
Pedigree and electropherogram findings of three limb-girdle muscular dystrophy type 1D patients. (A) Pedigree of patient 1. (B) Electropherogram presents a mutation in DNAJB6 genomic DNA (c.265T>A; p.Phe89Ile). (C) Novel mutation in patient 2 and his father (his mother's sequencing showed no variant in DNAJB6).
References
1. Iyadurai SJ, Kissel JT. The limb-girdle muscular dystrophies and the dystrophinopathies. Continuum (Minneap Minn). 2016; 22:1954–1977.

2. Harms MB, Sommerville RB, Allred P, Bell S, Ma D, Cooper P, et al. Exome sequencing reveals DNAJB6 mutations in dominantly-inherited myopathy. Ann Neurol. 2012; 71:407–416.

3. Ruggieri A, Brancati F, Zanotti S, Maggi L, Pasanisi MB, Saredi S, et al. Complete loss of the DNAJB6 G/F domain and novel missense mutations cause distal-onset DNAJB6 myopathy. Acta Neuropathol Commun. 2015; 3:44.

4. Couthouis J, Raphael AR, Siskind C, Findlay AR, Buenrostro JD, Greenleaf WJ, et al. Exome sequencing identifies a DNAJB6 mutation in a family with dominantly-inherited limb-girdle muscular dystrophy. Neuromuscul Disord. 2014; 24:431–435.

5. Sarparanta J, Jonson PH, Golzio C, Sandell S, Luque H, Screen M, et al. Mutations affecting the cytoplasmic functions of the co-chaperone DNAJB6 cause limb-girdle muscular dystrophy. Nat Genet. 2012; 44:450–455.

6. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424.

7. Palmio J, Jonson PH, Evilä A, Auranen M, Straub V, Bushby K, et al. Novel mutations in DNAJB6 gene cause a very severe early-onset limb-girdle muscular dystrophy 1D disease. Neuromuscul Disord. 2015; 25:835–842.

8. Sato T, Hayashi YK, Oya Y, Kondo T, Sugie K, Kaneda D, et al. DNAJB6 myopathy in an Asian cohort and cytoplasmic/nuclear inclusions. Neuromuscul Disord. 2013; 23:269–276.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download