Abstract
Coronary artery disease (CAD) is the leading cause of mortality worldwide, and various cardiovascular imaging modalities have been introduced for the purpose of diagnosing and determining the severity of CAD. More recently, advances in computed tomography (CT) technology have contributed to the widespread clinical application of cardiac CT for accurate and noninvasive evaluation of CAD. In this review, we focus on imaging assessment of CAD based upon CT, which includes coronary artery calcium screening, coronary CT angiography, myocardial CT perfusion, and fractional flow reserve CT. Further, we provide a discussion regarding the potential implications, benefits and limitations, as well as the possible future directions according to each modality.
Coronary artery disease (CAD) is the most prominent cause of death worldwide and contributes extensively to the economic burden of health care costs. To date, numerous cardiovascular imaging modalities designed to assess CAD have been introduced for clinical performance. Among those, much focus has been placed on computed tomography (CT)-based methods that include: coronary artery calcium (CAC) score, coronary CT angiography (CCTA), myocardial CT perfusion (CTP), fractional flow reserve derived from CT (FFRCT). The current review provides an overview regarding the benefits, limitations, and future directions of CT and discusses the appropriate use of CT in detecting CAD.
CAC is a well-known proxy of coronary artery atherosclerosis, and is closely associated with total atherosclerotic burden. CAC scoring is considered a robust measure for early screening of CAD, particularly in asymptomatic individuals. CAC is generally defined as a hyperattenuated lesion above a threshold of 130 Hounsfield units (HU) with an area ≥ 3 adjacent pixels on non-contrast enhanced cardiac CT.1) Though, there are several values of CAC cut-points that are often used for the purpose of stratification in categorizing the risk of CAD: 0 (very low), 1–99 (mild), 100–400 (moderate), > 400 (severe).
CAC has been demonstrated to be one of the most robust cardiovascular risk prediction markers. Indeed, numerous prospective studies have found that a moderate-to-high CAC likely reflects an incremental predictor of future obstructive CAD over and above conventional risk factors.2) Moreover, prior studies indicate that the addition of CAC to conventional risk factors also improves classification of cardiovascular risk.3) Conversely, a zero CAC score is closely associated with a low prevalence of adverse cardiac events and can be considered protective of CAD. Sarwar et al.4) evaluated the diagnostic and prognostic performance of a zero CAC score in asymptomatic and symptomatic individuals. In this cohort, only 146 of 25903 patients (0.45%) without CAC experienced a cardiovascular event in the study period, providing evidence that a zero CAC score is associated with a very low risk of future cardiovascular events. Additionally, risk prediction is not limited to coronary events; prior studies have demonstrated a moderate predictive benefit of CAC for incident stroke.5)
In the 2013 American College of Cardiology/American Heart Association guidelines, CAC was given a class IIb recommendation for assessment of cardiovascular risk in asymptomatic adults.6) CAC ≥ 300 may be taken into consideration, similar to family history, among patients with a 5–7.5% 10-year estimated risk of atherosclerotic cardiovascular disease using the newly developed pooled cohort equations. Although CAC score may reflect a strong tool for early detection of coronary atherosclerosis, several limitations should be considered. First, CAC is extremely limited when identifying the non-calcified plaque components of CAD.7) Thus, while the quantification of coronary calcium strongly prognosticates future adverse cardiovascular events, it may not reflect events that may result from plaques that are not yet calcified. Second, radiation exposure for CAC is low but non-negligible and must be considered carefully. The typical effective doses administered for CAC scanning are 1 mSv by electron beam CT and 3 mSv by multidetector row CT.8) Given these doses, continuous efforts have been made to reduce the amount of radiation dosages without jeopardizing the assessment of CAC score by techniques such as lower tube voltage and current.
Further, a zero CAC score has been contended by some to allow stratification of individuals whose risk is sufficiently low that pharmacological therapies could be avoided. For example, it has been estimated that current guidelines would recommend statins for the majority of the United States population over age 60.9) To this end, Nasir et al.10) evaluated within the Multi- Ethnic Study of Atherosclerosis (MESA) population the degree of risk reclassification for statin eligibility due to CAC. Of the patients who were recommended for high-intensity statins, 41% had CAC = 0 and had only 5.2 events over 1000 personyears, far below the 7.5% yearly event rate attributed to them using the pooled cohort equations. Similarly, Miedema et al.11) assessed the potential of CAC score for guiding aspirin use for the primary prevention of CAD in MESA. Participants with CAC score ≥ 100 had favorable risk/benefit estimates from aspirin use, while individuals with a zero CAC score would likely experience more harm than benefit. Finally, Bittencourt et al.12) evaluated the potential benefit of a polypill according to CAC score among the MESA participants. Individuals with a zero CAC score had a very low event rate with a high projected number needed to treat. These results suggest that avoidance of therapy in subjects with a zero CAC score could allow for significant reductions in the population considered for treatment and the number needed to treat for preventing CAD events, and could potentially improve safety and cost.
While current guidelines largely support CAC score in asymptomatic individuals, some investigators have maintained that CAC has a role as a potential gatekeeper to downstream procedures in symptomatic populations. Several studies provide insight regarding the incremental diagnostic and prognostic value of CAC score in symptomatic patients.13) In a study examining the prevalence and severity of CAD in 10037 patients with chest pain, a zero CAC score showed a negative predictive value (NPV) of 96% and 99% according to 50% and 70% coronary stenosis, respectively.13)
Patients undergoing lung cancer screening by thoracic nongated CT often demonstrate calcified coronary artery atherosclerosis and recently, the combined detection of CAD and lung cancer has been encouraged.14) In particular, the use of a one-time CT scan for identifying both CAC and lung cancer may display numerous benefits, including lower overall radiation exposure. Importantly, while there is generally high correlation between CAC and low dose lung cancer CT screening tests, there are practical differences in image acquisition and radiation dose between CAC scanning and low dose CT for lung cancer screening that relate to tube voltage and current, and electrocardiography (ECG) gating. To date, no study has evaluated the prognostic utility of CAC and low dose lung cancer screening CT when performed as a single exam, although such clinical trials are currently being designed.
CCTA is a non-invasive tool that can directly visualize the coronary anatomy with a reportedly high diagnostic accuracy, rendering it useful for anatomical assessment of CAD. Previous large prospective studies have reported that CCTA accurately identifies the presence and severity of obstructive CAD. For instance, the Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography (ACCURACY) trial was the first prospective multicenter trial to evaluate the diagnostic accuracy of CCTA in symptomatic patients without known CAD.15) Among 230 patients who underwent both CCTA and invasive coronary angiography (ICA), CCTA demonstrated high accuracy for detection of obstructive CAD with very high NPV (99%) for diagnosing patients with ≥ 70% stenosis. The Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography (CORE 64) study is a multicenter international trial using 64-slice CT detectors for identifying CAD in 291 patients with calcium scores ≤ 600.16) The sensitivity, specificity, positive predictive value (PPV) and NPV of ≥ 50% stenosis in CCTA were 85%, 90%, 91%, and 83%, respectively. In comparison to the ACCURACY study, where those with severely obstructive (≥ 70%) coronary stenosis represented only 13.9 percent of the study sample, the CORE 64 study observed a higher prevalence of obstructive CAD (defined at a lower threshold at ≥ 50% stenosis) at 56%, resulted in an expectedly higher PPV at the cost of NPV when compared to the ACCURACY trial results. A third multicenter trial studying the diagnostic value of 64 CCTA by Meijboom et al.17) documented the sensitivity, specificity, PPV and NPV for detecting patients with significant CAD (i.e., ≥ 50% lumen diameter reduction) was 99%, 64%, 86%, and 97%, respectively. This study reported a high sensitivity and NPV, similar to the ACCURACY trial. Importantly, both the ACCURACY trial and the study by Meijboom et al.17) enrolled only patients without known CAD, and suggests a very robust ability of CCTA to exclude obstructive CAD in this symptomatic population.15)16)
The prognostic value of CCTA for clinical outcomes has been well established by the Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter (CONFIRM) registry.18) Among 23854 patients without known CAD, the incidence of mortality was significantly associated with obstructive CAD according to a per-patient, per-vessel, and persegment analysis. Further still, the absence of CAD by CCTA was associated with a favorable prognosis (e.g., annualized death rate = 0.28%). To this end, CCTA may prove useful for predicting future mortality outcomes, and may also serve an important role for effectively ruling out future cardiovascular events.
Direct head-to-head comparisons of CCTA to the more traditional used stress tests have been recently evaluated. Recently, The Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial aimed to compare clinical outcomes in symptomatic patients that required further evaluation to initial strategy of anatomical testing by use of CCTA or functional testing.19) The adjusted hazard ratio for a CCTA strategy, as compared with a usual-care strategy of functional testing was 1.04 (95% confidence interval, 0.83 to 1.29), with adjustment for age, sex and several cardiovascular risk factors. In this study, there was a trend towards reduced rates of adverse clinical events at 12 months for individuals undergoing CCTA. To this end, the PROMISE trial indicates an initial strategy of CCTA performance is comparable to functional testing. Based upon this and similar data, current guidelines recommend the use of CCTA to symptomatic individuals, with a general focus on those considered low-to-intermediate risk.20) Societal guidance documents also endorse the use of CCTA in patients with discordant ECG exercise and imaging results, such as those who continue to experience symptoms even with a normal ECG exercise test.
While some studies have suggested a clinical benefit of CCTA when screening asymptomatic populations, others have reported conflicting results. In general, the prognostic implications of screening for occult CAD in asymptomatic individuals with CCTA, rather than CAC, have not been addressed adequately due to their very low rate of hard events.21)22) Cho et al.23) explored risk stratification by CCTA in 7590 patients without chest pain syndrome. Although both CAC score and CCTA significantly improved the performance of standard risk factor prediction models (likelihood ratio p < 0.05 for all), the additional riskpredictive advantage by CCTA did not appear to be clinically meaningful in the general asymptomatic population. In select high risk patients, CCTA may offer benefit over CAC; among 400 asymptomatic diabetic individuals without known CAD, segment stenosis by CCTA was associated with increased major adverse cardiovascular events after adjusting for numerous CAD risk factors as well as CAC score.22) Yet, the appropriate treatment of these individuals is still unknown. In the Screening for Asymptomatic Obstructive Coronary Artery Disease Among High-Risk Diabetic Patients Using CT Angiography, Following Core 64 (FACTOR-64), a randomized controlled trial consisting of asymptomatic diabetic patients with relatively high cardiovascular risk,24) 900 individuals were randomized to receive either screening with 64-slice CCTA or guideline-based optimal care. No differences in outcomes were observed between the CCTA and guideline-based strategy, although rates of adverse events were very low and thus may have lacked power to exclude a benefit to screening. Additional studies to improve our understanding of the utility of CCTA for risk stratification appear warranted.20)
CCTA enables assessment of atherosclerotic plaque volume and characteristics in a similar fashion to invasive intravascular ultrasound (IVUS). In a meta-analysis, CCTA proved to be highly accurate for determining plaque volume and area as compared with IVUS.25) Recently, atherosclerotic plaque characteristics assessed by CCTA have been shown to be associated with ischemic lesions identified by invasive fractional flow reserve (FFR), whereby positive remodeling and low attenuation plaques were independently associated with myocardial ischemia (odd ratio: 5.30, p < 0.001, and odd ratio: 2.1, p = 0.038, respectively).26) The latter findings suggest CCTA may be a useful adjunct for patient stratification and for improving specificity and PPV. Further, these atherosclerotic features identified by CCTA also provide predictive ability for future acute coronary syndromes and other adverse cardiovascular outcomes.27)
CCTA allows detection of anatomically obstructive coronary stenoses but, similar to anatomic evaluation by coronary angiography, cannot determine alone whether a stenosis is flow-limiting. In light of this, several prior studies have aimed to combine CCTA with established functional modalities, such as single photon emission CT (SPECT) or positron emission tomography myocardial perfusion imaging (MPI).28) Although these approaches are feasible, they are generally burdensome to patients who are required to undergo at least two tests, while paying a penalty to cost and radiation exposure. In this regard, combined CCTA and CTP imaging may benefit the patient by enabling both morphologic and functional assessment of CAD into a single technique with high accuracy.
The majority of studies for CTP have employed a static single-energy approach, with comparable diagnostic performance compared to SPECT or ICA with SPECT.29)30) Rochitte et al.30) assessed the diagnostic performance of integrated CT angiography (CTA) and CTP for identification of individuals with flow-limiting CAD, as defined by ICA with a concomitant perfusion deficit on SPECT in the first multicenter study. The patient- based diagnostic accuracy of the combined CTA-CTP was 87% in all patients, and increased further to 90% and 93% when patients without prior myocardial infarction and known CAD were excluded, respectively. Subsequently, this study concluded that the integration of CTA and CTP could correctly identify individuals with obstructive CAD defined as ≥ 50% stenosis by ICA causing a perfusion defect by SPECT.
Although recent advances in CT technology have allowed the assessment of CTP, there still exist significant limitations that constrain its widespread use in daily clinical practice. Artifacts from CTP are significant, and include beam hardening, misregistration, image noise and motion artifacts. Future improvements in CT technology might help to alleviate these artifactrelated problems. Others have appropriately contended that the additional cost and safety of additional contrast and radiation exposure should be continuously considered. Further, static CTP imaging is limited by the absence of quantitative techniques, but also by the acquisition of images for a single snap shot during the early arterial phase.31)
Dynamic myocardial CTP can overcome some flaws of static CTP and has emerged as a novel non-invasive imaging technique to evaluate reduced absolute myocardial blood flow. This technique has been demonstrated to have high diagnostic accuracy for the detection and exclusion of ischemic or infarcted myocardial segments.32) Although the majority of studies have been limited by study sample size, the diagnostic performance of dynamic myocardial CTP has been to date investigated in comparison with SPECT and ICA, invasive FFR, or cardiac magnetic resonance imaging (MRI).31)32)33) Ho et al.33) evaluated the ability of stress dynamic myocardial CTP for detecting abnormal blood flow reserve and infarction in comparison to stress nuclear MPI. The results showed that sensitivity, specificity, PPV, and NPV were 0.83, 0.78, 0.79, and 0.82, respectively, when compared with SPECT MPI. While compared with ICA, the results of dynamic CTP were 0.95, 0.65, 0.78, and 0.79, respectively. Recently, Bamberg et al.32) evaluated the use of dynamic CTP for the evaluation of myocardial ischemia in comparison to cardiac MRI. In that study, the diagnostic value of CT reported a sensitivity of 77.8%, specificity of 75.41%, NPV of 91.3%, and a modest PPV of 50.6%. Further, a higher diagnostic accuracy was observed for transmural perfusion defects and infarcted segments with a sensitivity of 87.8% and 85.3%, respectively. However, numerous potential limitations exist for dynamic CTP. Perhaps most importantly, the radiation dose of performing dynamic CTP is substantially higher compared to static CTP. Prior studies have reported average doses of ~20 mSv, equivalent to nearly 4–10 times CCTA doses. Also, the relatively long breath hold times and scan duration of 30 seconds that is necessary to complete the exam renders it difficult and uncomfortable to many patients. Furthermore, table movements may result in spatial misalignment. Thus, taking these points into consideration, additional studies along with further advances in CT technology are clearly needed to resolve these limitations.
Recently, application of computational fluid dynamics to typically acquired CCTA has allowed for a novel disruptive technology to calculate FFR from static CCTA data. FFRCT is utilized to assess the coronary anatomy without the need for additional radiation exposure, additional iodinated contrast, or administration of additional medications (Fig. 1). To date, several prospective multicenter trials have evaluated the diagnostic performance of FFRCT compared to an invasive FFR reference standard. The Diagnosis of ISChemia-Causing Stenoses Obtained Via NoninvasivE FRactional FLOW Reserve (DISCOVER- FLOW) study initially explored the diagnostic performance of FFRCT in 103 patients across 159 vessels.34) Anatomical obstruction was defined as a CCTA with stenosis ≥ 50%, and ischemia was defined using FFRCT with FFR ≤ 0.80. On a per-vessel analysis, the accuracy, sensitivity, specificity, PPV, and NPV were 84.3%, 87.9%, 82.2%, 73.9%, and 92.2%, respectively. Using receiver operating characteristic curve analysis, the area under the curve (AUC) was 0.80 for FFRCT, which was significantly higher than the AUC based on CCTA (e.g., 0.75, p < 0.001). A follow-up study–the Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography (DeFACTO) study–consisted of a larger population including 252 patients representing 407 vessels.35) Of these patients, 137 (54.4%) had an abnormal FFR determined by invasive FFR. On a per-patient basis, diagnostic accuracy, sensitivity, specificity, PPV, and NPV of FFRCT plus CCTA were 73%, 90%, 54%, 67%, and 84%, respectively. Interestingly, diagnostic accuracy of FFRCT in patients with lesions of intermediate stenosis (i.e., 30% to 70% stenosis) was significantly higher sensitivity than CCTA only (82% versus 37%), without a change in specificity. Recently, the Analysis of Coronary Blood Flow Using CT Angiography: Next Steps (NXT) study also demonstrated high diagnostic performance of FFRCT.36) Diagnostic accuracy, sensitivity, specificity, PPV, and NPV of FFRCT were 81%, 86%, 89%, 65%, and 93%, respectively. Compared with CCTA, FFRCT showed a significant reduction in the rate of false positives, particularly among those with lesions of intermediate stenosis (PPV: 63% versus 37%, p < 0.001). These prospective multicenter studies underscore the robustness of FFRCT to accurately pinpoint specific coronary lesions that cause ischemia, and extend the diagnostic paradigm beyond anatomically obstructive stenoses to lesion-specific ischemia.
Beyond improved diagnostic accuracy, FFRCT may also prove useful for guiding clinical decision-making. Using FFRCT to select patients for ICA and percutaneous coronary intervention (PCI) may lower health-related costs by 30% and may reduce the overall number of adverse cardiovascular events by 12% when compared to ICA or visual guidance for PCI.37) Recently, the Prospective LongitudinAl Trial of FFRCT: Outcome and resource iMpacts study (PLATFORM) trial evaluated the utility of FFRCT to improve patient selection for ICA among 584 patients with new onset chest pain. Patients who were randomized to FFRCT had cancellation of ICA in 61% of all patients, with normal ICA in only 12%, as compared to 73% of those randomized to usual care.38) One potentially intriguing application of FFRCT is its use to perform "virtual stenting" by computational modeling of coronary flow after virtual modification. Indeed, Kim et al.39) reported that there are a positive correlation between FFRCT and invasive FFR before and after stenting, with an overall accuracy of 96%. According to the latter study, treatment planning using FFRCT and 'virtual stenting' method can help to predict the therapeutic benefit of coronary revascularization prior to invasive coronary angiogram.
Recent developments in CT technology include dual-energy CT (DECT), a technique that allows for simultaneous or near-simultaneous imaging with two energy spectra. By exploiting the use of two photon energy levels, DECT creates a noticeable difference between 2 materials with regard to their energy-specific attenuation profile. In this way, DECT can provide beneficial information regarding tissue composition by using differences in energy-dependent attenuation of different tissues, which primarily is the distinguishing feature of DECT compared with single energy CT (SECT).40) To date, there are four methods of DECT that are practically available in the clinical setting that include: a dual-source dual-energy scanner (using two CT tubes and detectors), a single-source dual-energy scanner with fast kilovoltage switching (using a single CT tube and detector), a single- source dual-energy scanner with dual detector layers (using a single CT tube and 2 detector layers), and switching of energy levels between gantry rotations (Fig. 2).
Several studies have demonstrated the diagnostic accuracy of DECT performed during rest or during a stress protocol in comparison to other modalities such as SPECT, ICA, or cardiac MRI.41)42) Ruzsics et al.41) assessed the diagnostic performance of MPI at rest by using DECT among 36 patients compared with SPECT. The sensitivity and specificity values were 92%, 93%, respectively, with an accuracy of 93% for detecting a perfusion defect on SPECT. Most recently, Kim et al.42) evaluated the diagnostic value of adenosine-stress DECT using a secondgeneration (128-slice) dual-source CT (DSCT) for detecting myocardial perfusion defects compared with stress perfusion cardiac MRI in 50 patients. This study demonstrated a sensitivity and specificity of 77% and 94%, respectively, and the correlation between DECT perfusion and cardiac MRI was significantly positive (r = 0.602; p < 0.001).
Moreover, the DECT for ischemia determination compared to "gold standard" non-invasive and invasive techniques (DECIDE- Gold) trial was developed as a diagnostic performance study for testing rest-stress DECT angiography with perfusion.43) The primary purpose of this study is to evaluate the diagnostic accuracy of dual-energy CCTA combined with dual-energy CTP for assessing the hemodynamic significance of CAD non-invasively, using FFR as a reference standard. The DECIDEGold trial will help determine the diagnostic performance of dual-energy CTP for the identification and exclusion of hemodynamically significant coronary artery stenosis.
Prior invasive and pathological studies have identified several high-risk anatomic plaque characteristics fundamental to the pathogenesis of sudden cardiac death as well as acute coronary syndromes.27) These plaque features include plaque burden, thin-cap fibroatheroma, positive arterial remodeling, inflammatory infiltration, necrotic core, intraplaque hemorrhage, and spotty calcifications.44) Several studies using conventional SECT have determined the classification of coronary artery plaques based upon attenuation values measured by HU, and have demonstrated substantial overlap in HU between fibrousrich and lipid-rich components.45) Given that DECT images are acquired at two energy levels of photons, it has been considered that DECT may overcome these limitations. In a prospective study with tissue validation, Obaid et al.46) observed that DECT improves the differentiation of necrotic core and fibrous plaque in ex vivo postmortem arteries (i.e., sensitivity of 64%; specificity of 98% vs. sensitivity of 50%; specificity of 94%) in comparison with SECT. However, for in vivo analysis, the sensitivity to detect necrotic core when using DECT was lower than SECT (39% vs. 45%).
DECT has also been evaluated for its ability to lower overall contrast requirements, Raju et al.47) assessed the image quality and feasibility of a protocol with a reduced volume of iodinated contrast utilizing DECT angiography compared with a standard single-energy CCTA protocol. Although DECT angiography showed slightly inferior image quality, the signal-to-noise ratio and contrast-to-noise were comparable with the control group, with > 50% reduction in iodine dose. In this study, no differences were observed for overall radiation dose between SECT and DECT, with 2.31 ± 1.18 mSv for SECT and 2.23 ± 0.65 mSv for DECT, respectively.
Though DECT has recently seen a rapid progression in the field of cardiac CT, given the incipient stage for DECT, further studies are clearly required to validate its utility in clinical practice. By extension, the cost-effectiveness and clinical effectiveness based upon additional radiation exposure also warrants further consideration.
While CCTA has been well established as a highly accurate method for CAD detection and exclusion, several factors may limit the overall performance of CCTA, including relative tachycardias, arrhythmias, and/or high CAC levels. Thus these factors should be carefully considered prior to imaging procedures, given the effect they might impose on image quality and diagnostic accuracy, both of which are closely associated with spatial resolution and temporal resolution.
A newly introduced high definition CT (HDCT) scanner offers considerably improved in-plane spatial resolution to 0.23 mm, which is usually implemented with adaptive statistical iterative reconstruction to compensate for increased noise developed due to the higher spatial resolution of HDCT. Pontone et al.48) compared the image quality, diagnostic value, diagnostic accuracy, and radiation exposure of HDCT compared with standard definition CT (SDCT). HDCT demonstrated a higher image quality score (3.7 vs. 3.4, p < 0.001) and better overall diagnostic value (97% vs. 92%, p < 0.002) in calcified lesions. Moreover, the specificity, PPV, and accuracy were higher in the HDCT group compared with the SDCT group (e.g., 98%, 91%, and 99% vs. 95%, 80%, and 95%, respectively; p < 0.001).
Pitch in cardiac CT is defined as the ratio of table travel per gantry rotation to the X-ray beam. In currently used spiral or helical CT, pitch is associated with radiation dose and noise. A pitch value > 1 indicates gaps between radiation beams and reduced radiation exposure at the expense of providing a lower resolution, while a pitch < 1 implies overlapping of X-ray beam with a concomitant increase in radiation exposure.49) In singlesource CT, pitch is primarily limited to < 1.5 for guaranteeing gapless imaging.50) Notably, DSCT that uses two detectors and two X-ray tubes arranged at a 90° angle has enabled higher pitch value, which allows for a reduced radiation dose. Additionally, only one-quarter rotation is necessary to acquire one image because of the unique geometry of the DSCT device. As such, image gaps in the trajectory of the first detector as a result of rapid table motion are covered by the second detector. With this technique, the entire heart can be scanned during one cardiac cycle. Several previous studies have shown that a high pitch mode can effectively reduce radiation exposure (1.0 ± 0.3 mSv), albeit these studies were restricted to patients with regular or low heart rate (HR) below 65 beats/min.50) More recently, a third generation DSCT with high-pitch 192-slice CT can be performed at HR values up to 75 beats/min.
With advances in CT technology, 256-to-320 detector row CT have become available. These techniques have several advantages, including whole heart coverage resulting in contrast homogeneity and diminution of misregistration artifacts. Wong et al.51) compared the image quality of a second generation 320-detector row CT in patients with elevated HR compared with first generation 320-detector row CT. Compared with the first generation CT scanner, the second generation CT scanner was superior for better image quality (3.94 ± 0.6 vs. 3.45 ± 0.8, p = 0.001) and required a lower radiation dose (2.8 mSv vs. 4.3 mSv, p = 0.009) in individuals with a HR ≥ 65 beats/min.
Motion of the coronary arteries is the most common factor that restricts accurate interpretation of CCTA. Particularly, motion artifacts can become aggravated due to a high HR or irregular rhythms during the scanning process, which may significantly mitigate the diagnostic accuracy of CCTA. To date, various efforts to reduce motion artifacts in patients with high HR have been performed, such as the use of HR lowering medications, high-pitch CT, DSCT, and 320-detector row CT. More recently, a novel vendor-specific intra-cycle motion correction algorithm (MCA) (GE Healthcare, Waukasha, WI, USA) has been developed to control motion artifacts.52) This technique integrates image information from adjacent cardiac phases within a single cardiac cycle to characterize and compensate for coronary artery motion. Leipsic et al.52) reported the diagnostic accuracy and effect on image quality after implementing MCA were improved in participants who underwent CCTA without HR controlling medications. Andreini et al.53) assessed the diagnostic performance of MCA in conjunction with low-dose prospective ECG-triggering CCTA in patients with a pre-scanning HR > 70 beats/min or HR variability during scanning. Importantly, this study appeared to exclude individuals who initially presented with a very high HR and who were without HR-lowering therapy. In this regard, we await the findings from the Validation of an Intracycle CT Motion CORrection Algorithm for Diagnostic AccuracY (VICTORY) trial, a multicenter international study that will further elaborate as to whether MCA enhances the diagnostic value among persons undergoing CCTA who receive HR lowering medications.54)
In the recent past, cardiac CT has emerged as an extremely reliable tool for detection of CAD, with disruptive technologies such as FFRCT allowing for combined anatomic and physiologic assessment. In this review, we have provided a description of the benefits and disadvantages of several CCTA applications, having considered anatomic and physiologic assessment, as well as newer CT technologies (Table 1). Further, we have reviewed novel methods for improvement of image quality and radiation dose (Table 2). Continuing and future studies will help to define the clinical role of cardiac CT in the prevention and care of patients with cardiovascular disease.
Figures and Tables
Fig. 1
A 62-year-old Caucasian man visited due to exertional chest pain. A: Multiplanar reformat of a CT angiogram demonstrated a severe stenosis lesion in proximal LAD, a moderate to severe stenosis in middle LCX, severe stenosis in proximal RCA and suspected total occlusion in middle RCA. B: The FFRCT value of LAD, LCX, and RCA is < 0.50, 0.79, and 0.68, both of LAD and RCA indicated significant ischemia. The FFRCT value of LCX is borderline. All stenosis lesions in (A) was indicated by asterisks (*). LAD: left anterior descending coronary artery, LCX: left circumflex artery, RCA: right coronary artery, FFRCT: fraction flow reserve derived from CT.
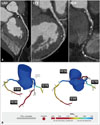
Fig. 2
Schematic illustration of 4 different approaches for obtaining dual-energy information. A: Dual source-detector pairs with each source operating at a different tube voltage. Each X-ray source covers a different scan field. B: Single source-detector pair with the source capable of rapid voltage switching in a single gantry rotation. C: Single source-detector pair with a dual-layer detector made of 2 different materials capable of differentiating between low-energy (upper layer) and high-energy (bottom layer) photons, with the source operating at constant tube voltage. D: Single source-detector pair with tube voltage switching between sequential gantry rotations. Fig. 2A, B, and C are courtesy of Philips Healthcare. Reprinted from Danad I et al. JACC Cardiovasc Imaging 2015;8:710-23, with permission of Elsevier.40)

Acknowledgements
This work was supported in part by grants from the National Heart, Lung, and Blood Institute (R01 HL111141, R01 HL115150, and R01 HL118019). The study was also funded, in part, by a generous gift from the Dalio Institute of Cardiovascular Imaging and the Michael Wolk Foundation.
References
1. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15:827–832.
2. Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012; 308:788–795.
3. Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004; 291:210–215.
4. Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009; 2:675–688.
5. Folsom AR, Kronmal RA, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008; 168:1333–1339.
6. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 129:25 Suppl 2. S49–S73.
7. Rubinshtein R, Gaspar T, Halon DA, Goldstein J, Peled N, Lewis BS. Prevalence and extent of obstructive coronary artery disease in patients with zero or low calcium score undergoing 64-slice cardiac multidetector computed tomography for evaluation of a chest pain syndrome. Am J Cardiol. 2007; 99:472–475.
8. Einstein AJ, Knuuti J. Cardiac imaging: does radiation matter? Eur Heart J. 2012; 33:573–578.
9. Pencina MJ, Navar-Boggan AM, D'Agostino RB Sr, Williams K, Neely B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014; 370:1422–1431.
10. Nasir K, Bittencourt MS, Blaha MJ, Blankstein R, Agatson AS, Rivera JJ, Miemdema MD, Sibley CT, Shaw LJ, Blumenthal RS, Budoff MJ, Krumholz HM. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015; 66:1657–1668.
11. Miedema MD, Duprez DA, Misialek JR, Blaha MJ, Nasir K, Silverman MG, Blankstein R, Budoff MJ, Greenland P, Folsom AR. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014; 7:453–460.
12. Bittencourt MS, Blaha MJ, Blankstein R, Budoff M, Vargas JD, Blumenthal RS, Agatston AS, Nasir K. Polypill therapy, subclinical atherosclerosis, and cardiovascular events-implications for the use of preventive pharmacotherapy: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2014; 63:434–443.
13. Villines TC, Hulten EA, Shaw LJ, Goyal M, Dunning A, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Lin FY, Maffei E, Raff GL, Min JK. CONFIRM Registry Investigators. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J Am Coll Cardiol. 2011; 58:2533–2540.
14. Hecht HS, Henschke C, Yankelevitz D, Fuster V, Narula J. Combined detection of coronary artery disease and lung cancer. Eur Heart J. 2014; 35:2792–2796.
15. Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008; 52:1724–1732.
16. Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008; 359:2324–2336.
17. Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, Nieman K, van Werkhoven JM, Pundziute G, Weustink AC, de Vos AM, Pugliese F, Rensing B, Jukema JW, Bax JJ, Prokop M, Doevendans PA, Hunink MG, Krestin GP, de Feyter PJ. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008; 52:2135–2144.
18. Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Maffei E, Raff G, Shaw LJ, Villines T, Berman DS. CONFIRM Investigators. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011; 58:849–860.
19. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL. PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015; 372:1291–1300.
20. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, Rubin GD; American College of Cardiology Foundation Appropriate Use Criteria Task Force; Society of Cardiovascular Computed Tomography; American College of Radiology; American Heart Association; American Society of Echocardiography; American Society of Nuclear Cardiology; North American Society for Cardiovascular Imaging; Society for Cardiovascular Angiography and Interventions; Society for Cardiovascular Magnetic Resonance, Kramer CM, Berman D, Brown A, Chaudhry FA, Cury RC, Desai MY, Einstein AJ, Gomes AS, Harrington R, Hoffmann U, Khare R, Lesser J, McGann C, Rosenberg A, Schwartz R, Shelton M, Smetana GW, Smith SC Jr. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010; 56:1864–1894.
21. Choi EK, Choi SI, Rivera JJ, Nasir K, Chang SA, Chun EJ, Kim HK, Choi DJ, Blumenthal RS, Chang HJ. Coronary computed tomography angiography as a screening tool for the detection of occult coronary artery disease in asymptomatic individuals. J Am Coll Cardiol. 2008; 52:357–365.
22. Min JK, Labounty TM, Gomez MJ, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan KM, Chow B, Cury R, Delago A, Dunning A, Feuchtner G, Hadamitzky M, Hausleiter J, Kaufmann P, Kim YJ, Leipsic J, Lin FY, Maffei E, Raff G, Shaw LJ, Villines TC, Berman DS. Incremental prognostic value of coronary computed tomographic angiography over coronary artery calcium score for risk prediction of major adverse cardiac events in asymptomatic diabetic individuals. Atherosclerosis. 2014; 232:298–304.
23. Cho I, Chang HJ, Sung JM, Pencina MJ, Lin FY, Dunning AM, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Callister TQ, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Maffei E, Cademartiri F, Kaufmann P, Shaw LJ, Raff GL, Chinnaiyan KM, Villines TC, Cheng V, Nasir K, Gomez M, Min JK. CONFIRM Investigators. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry). Circulation. 2012; 126:304–313.
24. Muhlestein JB, Lappé DL, Lima JA, Rosen BD, May HT, Knight S, Bluemke DA, Towner SR, Le V, Bair TL, Vavere AL, Anderson JL. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA. 2014; 312:2234–2243.
25. Fischer C, Hulten E, Belur P, Smith R, Voros S, Villines TC. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: a meta-analysis. J Cardiovasc Comput Tomogr. 2013; 7:256–266.
26. Park HB, Heo R, ó Hartaigh B, Cho I, Gransar H, Nakazato R, Leipsic J, Mancini GB, Koo BK, Otake H, Budoff MJ, Berman DS, Erglis A, Chang HJ, Min JK. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging. 2015; 8:1–10.
27. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000; 20:1262–1275.
28. Di Carli MF, Dorbala S, Curillova Z, Kwong RJ, Goldhaber SZ, Rybicki FJ, Hachamovitch R. Relationship between CT coronary angiography and stress perfusion imaging in patients with suspected ischemic heart disease assessed by integrated PET-CT imaging. J Nucl Cardiol. 2007; 14:799–809.
29. Ko BS, Cameron JD, Meredith IT, Leung M, Antonis PR, Nasis A, Crossett M, Hope SA, Lehman SJ, Troupis J, DeFrance T, Seneviratne SK. Computed tomography stress myocardial perfusion imaging in patients considered for revascularization: a comparison with fractional flow reserve. Eur Heart J. 2012; 33:67–77.
30. Rochitte CE, George RT, Chen MY, Arbab-Zadeh A, Dewey M, Miller JM, Niinuma H, Yoshioka K, Kitagawa K, Nakamori S, Laham R, Vavere AL, Cerci RJ, Mehra VC, Nomura C, Kofoed KF, Jinzaki M, Kuribayashi S, de Roos A, Laule M, Tan SY, Hoe J, Paul N, Rybicki FJ, Brinker JA, Arai AE, Cox C, Clouse ME, Di Carli MF, Lima JA. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J. 2014; 35:1120–1130.
31. Bamberg F, Becker A, Schwarz F, Marcus RP, Greif M, von Ziegler F, Blankstein R, Hoffmann U, Sommer WH, Hoffmann VS, Johnson TR, Becker HC, Wintersperger BJ, Reiser MF, Nikolaou K. Detection of hemodynamically significant coronary artery stenosis: incremental diagnostic value of dynamic CT-based myocardial perfusion imaging. Radiology. 2011; 260:689–698.
32. Bamberg F, Marcus RP, Becker A, Hildebrandt K, Bauner K, Schwarz F, Greif M, von Ziegler F, Bischoff B, Becker HC, Johnson TR, Reiser MF, Nikolaou K, Theisen D. Dynamic myocardial CT perfusion imaging for evaluation of myocardial ischemia as determined by MR imaging. JACC Cardiovasc Imaging. 2014; 7:267–277.
33. Ho KT, Chua KC, Klotz E, Panknin C. Stress and rest dynamic myocardial perfusion imaging by evaluation of complete time-attenuation curves with dual-source CT. JACC Cardiovasc Imaging. 2010; 3:811–820.
34. Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, Dunning A, DeFrance T, Lansky A, Leipsic J, Min JK. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011; 58:1989–1997.
35. Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C, Erglis A, Lin FY, Dunning AM, Apruzzese P, Budoff MJ, Cole JH, Jaffer FA, Leon MB, Malpeso J, Mancini GB, Park SJ, Schwartz RS, Shaw LJ, Mauri L. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012; 308:1237–1245.
36. Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, Jensen JM, Mauri L, De Bruyne B, Bezerra H, Osawa K, Marwan M, Naber C, Erglis A, Park SJ, Christiansen EH, Kaltoft A, Lassen JF, Bøtker HE, Achenbach S. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol. 2014; 63:1145–1155.
37. Hlatky MA, Saxena A, Koo BK, Erglis A, Zarins CK, Min JK. Projected costs and consequences of computed tomography-determined fractional flow reserve. Clin Cardiol. 2013; 36:743–748.
38. Douglas PS, Pontone G, Hlatky MA, Patel MR, Norgaard BL, Byrne RA, Curzen N, Purcell I, Gutberlet M, Rioufol G, Hink U, Schuchlenz HW, Feuchtner G, Gilard M, Andreini D, Jensen JM, Hadamitzky M, Chiswell K, Cyr D, Wilk A, Wang F, Rogers C, De Bruyne. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFRCT: outcome and resource impacts study. Eur Heart J. 2015; 36:3359–3367.
39. Kim KH, Doh JH, Koo BK, Min JK, Erglis A, Yang HM, Park KW, Lee HY, Kang HJ, Kim YJ, Lee SY, Kim HS. A novel noninvasive technology for treatment planning using virtual coronary stenting and computed tomography-derived computed fractional flow reserve. JACC Cardiovasc Interv. 2014; 7:72–78.
40. Danad I, Fayad ZA, Willemink MJ, Min JK. New applications of cardiac computed tomography: dual-energy, spectral, and molecular CT imaging. JACC Cardiovasc Imaging. 2015; 8:710–723.
41. Ruzsics B, Schwarz F, Schoepf UJ, Lee YS, Bastarrika G, Chiaramida SA, Costello P, Zwerner PL. Comparison of dual-energy computed tomography of the heart with single photon emission computed tomography for assessment of coronary artery stenosis and of the myocardial blood supply. Am J Cardiol. 2009; 104:318–326.
42. Kim SM, Chang SA, Shin W, Choe YH. Dual-energy CT perfusion during pharmacologic stress for the assessment of myocardial perfusion defects using a second-generation dual-source CT: a comparison with cardiac magnetic resonance imaging. J Comput Assist Tomogr. 2014; 38:44–52.
43. Truong QA, Knaapen P, Pontone G, Andreini D, Leipsic J, Carrascosa P, Lu B, Branch K, Raman S, Bloom S, Min JK. Rationale and design of the dual-energy computed tomography for ischemia determination compared to “gold standard” non-invasive and invasive techniques (DECIDEGold): a multicenter international efficacy diagnostic study of rest-stress dual-energy computed tomography angiography with perfusion. J Nucl Cardiol. 2015; 22:1031–1040.
44. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006; 47:8 Suppl. C13–C18.
45. Becker CR, Nikolaou K, Muders M, Babaryka G, Crispin A, Schoepf UJ, Loehrs U, Reiser MF. Ex vivo coronary atherosclerotic plaque characterization with multi-detector-row CT. Eur Radiol. 2003; 13:2094–2098.
46. Obaid DR, Calvert PA, Gopalan D, Parker RA, West NE, Goddard M, Rudd JH, Bennett MR. Dual-energy computed tomography imaging to determine atherosclerotic plaque composition: a prospective study with tissue validation. J Cardiovasc Comput Tomogr. 2014; 8:230–237.
47. Raju R, Thompson AG, Lee K, Precious B, Yang TH, Berger A, Taylor C, Heilbron B, Nguyen G, Earls J, Min J, Carrascosa P, Murphy D, Hague C, Leipsic JA. Reduced iodine load with CT coronary angiography using dual-energy imaging: a prospective randomized trial compared with standard coronary CT angiography. J Cardiovasc Comput Tomogr. 2014; 8:282–288.
48. Pontone G, Bertella E, Mushtaq S, Loguercio M, Cortinovis S, Baggiano A, Conte E, Annoni A, Formenti A, Beltrama V, Guaricci AI, Andreini D. Coronary artery disease: diagnostic accuracy of CT coronary angiography--a comparison of high and standard spatial resolution scanning. Radiology. 2014; 271:688–694.
49. Primak AN, McCollough CH, Bruesewitz MR, Zhang J, Fletcher JG. Relationship between noise, dose, and pitch in cardiac multi-detector row CT. Radiographics. 2006; 26:1785–1794.
50. Stolzmann P, Goetti RP, Maurovich-Horvat P, Hoffmann U, Flohr TG, Leschka S, Alkadhi H. Predictors of image quality in high-pitch coronary CT angiography. AJR Am J Roentgenol. 2011; 197:851–858.
51. Wong DT, Soh SY, Ko BS, Cameron JD, Crossett M, Nasis A, Troupis J, Meredith IT, Seneviratne SK. Superior CT coronary angiography image quality at lower radiation exposure with second generation 320-detector row CT in patients with elevated heart rate: a comparison with first generation 320-detector row CT. Cardiovasc Diagn Ther. 2014; 4:299–306.
52. Leipsic J, Labounty TM, Hague CJ, Mancini GB, O'Brien JM, Wood DA, Taylor CM, Cury RC, Earls JP, Heilbron BG, Ajlan AM, Feuchtner G, Min JK. Effect of a novel vendor-specific motion-correction algorithm on image quality and diagnostic accuracy in persons undergoing coronary CT angiography without rate-control medications. J Cardiovasc Comput Tomogr. 2012; 6:164–171.
53. Andreini D, Pontone G, Mushtaq S, Bertella E, Conte E, Segurini C, Baggiano A, Bartorelli AL, Annoni A, Formenti A, Petullà M, Beltrama V, Fiorentini C, Pepi M. Low-dose CT coronary angiography with a novel IntraCycle motion-correction algorithm in patients with high heart rate or heart rate variability. Eur Heart J Cardiovasc Imaging. 2015; 16:1093–1100.
54. Min JK, Arsanjani R, Kurabayashi S, Andreini D, Pontone G, Choi BW, Chang HJ, Lu B, Narula J, Karimi A, Roobottom C, Gomez M, Berman DS, Cury RC, Villines T, Kang J, Leipsic J. Rationale and design of the ViCTORY (Validation of an Intracycle CT Motion CORrection Algorithm for Diagnostic AccuracY) trial. J Cardiovasc Comput Tomogr. 2013; 7:200–206.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download