Abstract
Purpose
The purpose of the present study was to investigate whether hardness of liver surface correlated with degree of liver fibrosis, and its association with posthepatectomy liver failure (PHLF).
Methods
A shore durometer was used to measure hepatic hardness in 41 patients with hepatocellular carcinoma (HCC) and in 10 patients with normal liver. We investigated how hepatic hardness correlates with various values indicating the degree of liver fibrosis, and how it correlates with PHLF.
Results
In the normal liver group, the surface shore units (SU) was 15.06 ± 2.64. In the HCC group, there was a correlation between surface SU and preoperative results indicating liver fibrosis. Among patients with PHLF after resecting over 3 segments, the surface SU of patients with grade A PHLF was 21.85 ± 5.63, and the surface SU of patients with grade C PHLF was 35.75 ± 9.26. In patients with PHLF after resecting 2 or less segments, the surface SU of patients with PHLF grade A was 20.95 ± 5.18, and the surface SU of patients with PHLF grade B was 31.60 ± 5.57. In predicting PHLF, surface SU was more effective than preoperative platelet count, spleen volume, or liver fibrosis index.
Conclusion
Hepatic hardness measured by the shore durometer was correlated with the degree of liver fibrosis. Liver surface SU was a more effective parameter for predicting PHLF, as compared to other indicators evaluated before hepatectomy. The decision to perform major hepatectomy should be reconsidered in cases with a liver surface SU of >30.
Various methods are available for measuring liver function and degree of liver fibrosis before hepatectomy, but currently, there are no methods that can be performed during surgery. In addition, there are no established methods to quantify the degree of cirrhosis in the operating room. Shore durometer is an instrument for measuring the hardness of objects. It has an indentation body on the working face, which is connected by a spring. The spring measuring force of this device was converted to shore units (SU), an arbitrary parameter of hardness. In the case of pancreas, measuring the hardness of pancreas parenchyma using a shore durometer has been reported [1234].
Despite the possible relationship between hepatic hardness and degree of cirrhosis, this has yet to be proven, because hepatic hardness is not measured quantitatively. In addition, the correlation between quantitative hepatic hardness and posthepatectomy liver failure (PHLF) has not been evaluated.
The primary objective of the present study was to determine the correlation of hepatic hardness with preoperative tests for liver function and postoperative pathological fibrosis. The second objective was to evaluate whether hepatic hardness was associated with PHLF.
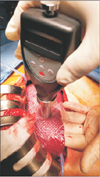
From November 1, 2015 to December 31, 2016, 41 patients with hepatocellular carcinoma (HCC) and 10 patients with normal liver who underwent surgery were retrospectively studied. Because shore durometer was used as a measuring device, and it did not harm the patients or affect the treatment direction, Incheon St. Mary's Hospital (OC17RESI0039) Institutional Review Board approved the study. All patients in the normal liver group were confirmed as without hepatic fibrosis in the postoperative pathology report. Preoperative indocyanine green retention test at 15 minutes (ICG-r15), liver fibrosis index (LFI) by real-time elastography (RTE), CT with spleen volumetry, Child-Turcotte-Pugh (CTP) score, and model for endstage liver disease (MELD) score were checked in HCC patients, but the ICGr-15 and LFI were not obtained in normal liver patients. RTE was performed using a Hitachi Avius device (Hitachi Medical, Tokyo, Japan) and a linear probe (EUP-L52; central frequency, 5.5 MHz). The linear probe was placed on the right lobe of the liver through an intercostal space, with the patient in supine position. A rectangular area measuring 30 mm in length and 20 mm in breath and 10 mm below the surface of liver, which was free from large vessels was selected. To obtain good images, scanning was performed to avoid large vessels and attenuation by the lungs and ribs. The mean LFI was determined from 10 images according to a technique described earlier [56]. Spleen volumetric analysis was performed using OsiriX version 5.0.2 32-bit (Pixmeo, Geneva, Switzerland), as described previously [7]. Hepatic hardness was measured using a handheld shore durometer (Guangzhou Landtek Instruments, Shore Hardness Tester HT-6510OO, Guangzhou, China). We used the ASTM D2240-00 type OO shore durometer, with a scale of 0 to 100 SU, with higher values indicating harder tissue. In the case of open surgery, a thin transparent film was applied on the liver surface, and the shore durometer was pressed perpendicularly on the liver surface (Fig. 1). The number on the instrument panel was determined as hepatic hardness, and a mean value was obtained from measuring 10 times at different sites. Although the thickness of the left liver is thinner than that of the right liver, if the thickness of the liver is 1 cm or more, there is no difference according to the left and right sides to measure. Specimen SU values were obtained from the excised specimen in the same manner. The extent of hepatectomy was determined by considering the ICG-r15, tumor size and location [8]. In laparoscopic hepatectomy, hepatic hardness was measured only in the excised specimen and in radiofrequency ablation (RFA) cases, only in the liver surface. We divided the patients into 3 groups based on the extent of hepatic resection. Group 1 was defined as patients with resection of more than 3 segments; group 2 as patients with 2 or less segments removed; and group 3, as patients in whom only RFA was performed. All excised liver tissue was categorized by pathologic fibrosis using the METAVIR scoring system after surgery. In the postoperative period, all patients were classified based on the grading of PHLF. Grade A is PHLF resulting in abnormal laboratory parameters but requiring no change in the clinical management of the patient. Grade B is PHLF resulting in a deviation from the regular clinical management but manageable without invasive treatment; and grade C is PHLF resulting in a deviation from the regular clinical management and requiring invasive treatment [9].
SU values were compared across various preoperative parameters by using Mann-Whitney or Kruskal-Wallis tests. The Pearson correlation coefficient was used for correlation between liver surface and specimen SU and preoperative parameters. All statistical analyses were conducted using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). A P-value of <0.05 was considered as statistical significance.
Of the 10 patients in the normal liver group, there were 4 male patients and 6 female patients with a mean age of 63.8 years (range, 54–81 years). All patients were diagnosed with metastatic colon cancer, and underwent hepatectomy for liver metastasis. There were no abnormalities on preoperative liver function and no abnormal findings on postoperative liver pathology. Of the 41 patients in the HCC group, there were 36 male patients and 5 female patients with a mean age of 60.4 years (range, 30–83 years); and 30 patients (73.1%) had HBV, 3 (7.3%) had HCV, and 8 (19.6%) had alcoholic hepatitis. Based on the extent of hepatic resection, there were 8 patients (19.5%) in group 1, 29 patients (70.7%) in group 2, and 4 patients (9.8%) in group 3. The average values of the SU measured in the normal liver group were 15.06 ± 2.64 on the liver surface, and 22.59 ± 3.57 on the excised specimen (P < 0.001). The average values of SU in the HCC group were 24.98 ± 7.44 on the liver surface and 35.14 ± 9.74 on the excised specimen (P < 0.001). As ICGr15 increased, the surface SU and specimen SU both showed an increasing trend, without statistical significance (P = 0.142, P = 0.883, respectively). As the CTP score increased, surface SU increased (P = 0.02). However, in some cases, the specimen SU could not be measured because RFA was performed in all patients with a CTP score of 6. The SU according to MELD score and METAVIR score showed a similar pattern. However, there was no correlation between SU and LFI (Table 1). Preoperative serum albumin, platelet count, PT and spleen volume were associated with surface SU. The LFI test was used to evaluate liver fibrosis before surgery. However, there was no significant correlation between LFI and SU in this study (Table 2). Moreover, preoperative serum albumin was associated with LFI, but other parameters showed no correlation with LFI (Table 3).
Among the 8 patients in group 1, there were 6 patients with grade A PHLF. In these patients, the surface SU was 21.85 ± 5.63 and the specimen SU was 32.24 ± 5.80. The remaining two patients in group 1 had grade C PHLF, with a surface SU of 35.75 ± 9.26 and a specimen SU of 63.35 ± 5.44. There was a trend toward a difference between PHLF grade A and grade C surface SU and specimen SU, although there was no statistical difference because of the small number of patients. Of the 29 patients in group 2, the surface SU of patients with grade A and B PHLF were 20.95 ± 5.18 and 31.60 ± 5.57, respectively (P = 0.018). The specimen SU of patients with grade A and B PHLF were 31.21 ± 6.05 and 41.51 ± 4.57, respectively (P < 0.001). And LFI of grade A and B PHLF were 3.53 ± 0.61 and 4.35 ± 0.88, respectively (P = 0.016). However, there was no difference in preoperative platelet count and spleen volume between grade A and B PHLF. Among the four patients in group 3, the surface SU of PHLF grade A and B were 25.20 and 33.47 ± 1.62, respectively, with no significant difference because of the small number of patients (Table 4).
A 60-year-old male patient underwent right hemihepatectomy with a 13-cm-sized hepatitis B-related HCC. The patient's preoperative total bilirubin was 1.0 mg/dL; ICGR15, 4%; platelet count, 235,000/µL; PT (international normalized ratio, INR), 1.12; CTP score, 5; spleen volume, 177.96 cm3; LFI, 4.42; and expected remnant liver volume, 39% in CT volumetry. The intraoperative surface SU was 42.3. We performed a right hemihepatectomy, which was uneventful. However, the patient deteriorated into hepatic failure and underwent liver transplantation.
An 81-year-old male patient underwent right hemihepatectomy for a 15-cm-sized hepatitis Brelated HCC. The patient's preoperative total bilirubin was 1.0 mg/dL; ICGR15, 8%; platelet count, 292,000/µL; PT (INR), 1.13; CTP score, 5; spleen volume, 305.84 cm3; LFI, unchecked; and expected remnant liver volume, 35% in CT volumetry. The remnant liver volume was considered as sufficient because of the extensive HCC included in the resected liver. The intraoperative surface SU was 29.2. We performed right hemihepatectomy uneventfully. However, hepatic failure ensued, and the patient died.
Despite improvement in preoperative evaluation methods, surgical technique, and perioperative management, surgeons still experience cases with mortality due to severe PHLF. In addition, when emergency hepatectomy due to HCC rupture or trauma is required, the condition of the cirrhotic liver can only be predicted visually in the operating room. By experience, skilled surgeons can visually confirm the degree of liver cirrhosis, and predict the likelihood of PHLF, but this is an inaccurate approach that cannot be expressed numerically.
The shore durometer is a device to measure hardness. It is noninvasive, has the advantage of producing results immediately, and is easy to use. The shore durometer has several scales based on the degree of hardness. We used an ASTM D2240-00 type OO shore durometer because we think it is the most suitable type to measure the hardness of human soft tissue, especially liver. And in the case of this type of durometer, the spring connected to the indentation body on the working face is soft and does not cause liver injury.
Our present study was designed to quantitatively measure hepatic hardness and determine it correlation with preoperative results, and to predict PHLF. The results indicated that hepatic hardness was correlated with the preoperative tests. There was a difference between surface SU and specimen SU, presumably because hepatic hardness was changed more softly by blood and body fluid in the tissues. However, specimen SU showed no significant correlation with preoperative tests, possibly due to the inconsistent amounts of blood and body fluid contained within the resected specimen. Therefore, surface SU may be more accurate in measuring hepatic hardness. The measured mean surface SU of the normal liver in our study was 15.06 ± 2.64. The surface SU was more effective in predicting PHLF than preoperative platelet count, spleen volume, and LFI. In the case of SU of approximately 30, limited resection caused grade B PHLF, but not grade C. However, major hepatectomy such as resection over four segments, resulted in severe hepatic failure in patients with SU > 30; and one patient died. In the mortality patient, the SU was not > 30 (29.2); however, the old age of the patient may have contributed to the severe hepatic failure. A study that includes a larger number of cases is required to establish a cutoff surface SU value based on patients' age that ensures safe hepatectomy.
In group 1, the difference between the surface SU and the specimen SU in the PHLF grade C patients was 27.6 ± 3.82, which was much higher than that of the other groups (9.32 ± 3.82). However, there was no statistical significance because there were only 2 patients.
Based on a previous report, LFI using RTE and fibroscan are suitable for assessing liver fibrosis and cirrhosis severity [6101112]. However, in this study, no association of LFI with preoperative parameters indicating liver fibrosis was detected, possibly due to absence of differences in the preoperative parameters since we only included patients considering hepatectomy. LFI and SU did not show a significant correlation in this study, possibly due to the narrow range of patient selection. Nevertheless, LFI is considered a good method for predicting PHLF.
The shore durometer is a useful tool to determine the liver fibrosis status from the viewpoint of the surgeon. In addition, there is an advantage in predicting PHLF compared to other methods before surgery, but it is difficult to measure before surgery and current device has a disadvantage that it is difficult to measure in patients with laparoscopic hepatectomy. And a study that includes a larger number of cases is required to confirm these results.
In conclusion, hepatic hardness measured by the shore durometer showed correlation with the degree of liver fibrosis in hepatectomy patients. Liver surface SU was a more powerful tool for predicting PHLF than any other indicator measured before hepatectomy. The decision to perform major hepatectomy such as resection over four segments should be reconsidered in cases with a liver surface SU of > 30.
Figures and Tables
References
1. Belyaev O, Herden H, Meier JJ, Muller CA, Seelig MH, Herzog T, et al. Assessment of pancreatic hardness-surgeon versus durometer. J Surg Res. 2010; 158:53–60.
2. Belyaev O, Polle C, Herzog T, Munding J, Chromik AM, Meurer K, et al. Effects of intra-arterial octreotide on pancreatic texture: a randomized controlled trial. Scand J Surg. 2013; 102:164–170.
3. Belyaev O, Rosenkranz S, Munding J, Herzog T, Chromik AM, Tannapfel A, et al. Quant itat ive assessment and determinants of suture-holding capacity of human pancreas. J Surg Res. 2013; 184:807–812.
4. Foitzik T, Gock M, Schramm C, Prall F, Klar E. Octreotide hardens the pancreas. Langenbecks Arch Surg. 2006; 391:108–112.
5. Tatsumi C, Kudo M, Ueshima K, Kitai S, Ishikawa E, Yada N, et al. Non-invasive evaluation of hepatic fibrosis for type C chronic hepatitis. Intervirology. 2010; 53:76–81.
6. Kim YW, Kwon JH, Jang JW, Kim MJ, Oh BS, Chung KW, et al. Diagnostic usefulness of real-time elastography for liver fibrosis in chronic viral hepatitis B and C. Gastroenterol Res Pract. 2014; 2014:210407.
7. van der Vorst JR, van Dam RM, van St iphout RS, van den Broek MA, Hollander IH, Kessels AG, et al. Virtual liver resection and volumetric analysis of the future liver remnant using open source image processing software. World J Surg. 2010; 34:2426–2433.
8. Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993; 9:298–304.
9. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011; 149:713–724.
10. Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003; 29:1705–1713.
11. Feng YH, Hu XD, Zhai L, Liu JB, Qiu LY, Zu Y, et al. Shear wave elastography results correlate with liver fibrosis histology and liver function reserve. World J Gastroenterol. 2016; 22:4338–4344.
12. Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006; 55:403–408.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download