Abstract
Purpose
Liver volumetry is a vital component in living donor liver transplantation to determine an adequate graft volume that meets the metabolic demands of the recipient and at the same time ensures donor safety. Most institutions use preoperative contrast-enhanced CT image-based software programs to estimate graft volume. The objective of this study was to evaluate the accuracy of 2 liver volumetry programs (Rapidia vs. Dr. Liver) in preoperative right liver graft estimation compared with real graft weight.
Methods
Data from 215 consecutive right lobe living donors between October 2013 and August 2015 were retrospectively reviewed. One hundred seven patients were enrolled in Rapidia group and 108 patients were included in the Dr. Liver group. Estimated graft volumes generated by both software programs were compared with real graft weight measured during surgery, and further classified into minimal difference (≤15%) and big difference (>15%). Correlation coefficients and degree of difference were determined. Linear regressions were calculated and results depicted as scatterplots.
Results
Minimal difference was observed in 69.4% of cases from Dr. Liver group and big difference was seen in 44.9% of cases from Rapidia group (P = 0.035). Linear regression analysis showed positive correlation in both groups (P < 0.01). However, the correlation coefficient was better for the Dr. Liver group (R2 = 0.719), than for the Rapidia group (R2 = 0.688).
Liver transplantation is the gold standard treatment for patients with end-stage liver disease. Since the report of a first successful adult living donor liver transplantation (LDLT), its practice has steadily increased in countries where living donors are practically the only source of organs due to the scarcity of cadaver donors [12345]. The use of the right liver for adult recipients has also become routine in most institutions.
Liver volumetry is a vital component in the evaluation of potential living liver donors because a liver remnant volume of 30%–40% of the total liver volume (TLV) is required for donor safety [6]. The most common reason for donor exclusion based on imaging is inadequate liver volume. The choice of liver graft harvesting (whether right or left lobe) is also dependent on donor liver volume. To prevent small-for-size syndrome (SFSS) in recipients, a graft-to-recipient weight ratio of at least 0.8% or a graft-to-standard liver volume ratio of at least 40% is needed for adequate graft function [7]. SFSS can be defined as either graft dysfunction or nonfunction during the 1st postoperative week evidenced by cholestasis, ascites, coagulopathy, and encephalopathy after exclusion of other etiologies [8].
The estimation of liver volume can be determined by noninvasive CT scan-based regression and image processing approaches using anthropometric dimensions. A standard technique of manual tracing of liver boundaries and summation of liver area from a standard grid provided on the roentgenogram is used for the definition of liver volume on individual CT images. However, this process may be subjective due to intra/interobserver variation. The method is also time-consuming and usually takes about 25–40 minutes to complete [910].
Since the early 1990's, various semiautomated and automated, computer software-based liver volumetry and segmentation techniques have been developed and yielded comparable liver volumes with the manual approach. With the use of thresholding, morphologic filtering, feature analysis, region growing, probabilistic models, and conversion factors, these softwares provided good estimation of liver volumes in a shorter period of time [9101112131415161718]. Table 1 summarizes the comparison between the types of available liver volumetry softwares.
However, there have been only a limited number of reports in literature regarding the accuracy of these softwares. The objective of this study was to evaluate the accuracy of two liver volumetry programs (Rapidia vs. Dr. Liver) in preoperative graft estimation compared with real graft weight.
This is a retrospective study that compared a manual program for liver volumetry with a semiautomated software, using the real graft weight of right liver during donor operation as standard, in LDLT.
This study was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (approval number: 1607-066-776) and the requirement for informed consent was waived.
From October 2013 to August 2015, a total of 421 cases of liver transplantation were performed at Seoul National University Hospital (Seoul, Korea). One hundred forty-seven cases were deceased donor transplantations and 274 cases were living donor transplantations. We reviewed the adult living donor cases from the medical records and consecutively enrolled them in a retrospective database. Data from cases such as pediatric - 29, left lobe donation - 22 and 8 more cases, where some data were missing were excluded from our study. Finally, 215 patients in whom both the preoperative volumetry data and real graft weight information were available were subjected for data analysis.
Patients were divided into 2 groups, those in which our institution exclusively used Rapidia software (Infinitt Co., Ltd., Seoul, Korea) for preoperative volumetry from October 2013 to April 2014, and those in which we exclusively used Dr. Liver software (Virtual Liver Surgery Planning System, Humanopia Co. Ltd, Pohang, Korea) from April 2014 to August 2015. One hundred seven patients were enrolled in Rapidia group and 108 patients were included in the Dr. Liver group.
At our institution, a multidetector row helical computed tomography (MDCT) using a standard protocol for multiphasic liver CT imaging (reconstruction section thickness of 2.5–3 mm) is routinely used for preoperative imaging evaluation of all LDLT donors.
Axial images were loaded to a computer workstation where the semimanual Rapidia software was installed. The liver extraction was performed as described by Jung et al. [17]. Cross-sectional areas of the liver on each transverse sliced image were obtained by manually tracing the contour of the liver using an electronic cursor. The free-curves were drawn by three experienced surgeons. The voxels/pixels to be included in a region of interest were then identified, based on the level or range of thresholding determined and adjusted by the surgeons to allow the software to include most of liver parenchyma (especially in cases of fatty liver). Liver parenchymal volume was then automatically generated by summation of the manually calculated intraboundary areas of successive transverse sliced images [18].
The arterial, portal and venous phase series of images from MDCT scans were used. Volumetric analysis was performed using the fast and accurate semi-automatic algorithm for liver extraction developed by Yang et al. [19]. The steps included denoising of CT images with an anisotropic diffusion filter, selecting multiple seed points on 5 or 6 slices, detection of initial liver area by a fast marching level set method, and propagation of the initially detected liver area to reach liver boundaries. Extraction of the portal vein, hepatic vein, and inferior vena cava (IVC) were done using the same process. The gallbladder, IVC, blood, and other vascular and biliary structures were excluded in the liver volume calculation. The middle hepatic vein was used as a guide for the transection line of the virtual liver resection but was excluded from the virtual resection area. The TLV and right lobe volume were then calculated automatically.
During bench surgery, the right liver lobe was flushed with an organ perfusion solution (HTK solution, Custodiol, Kohler Pharma, Alsbach, Germany) cooled to 4℃ through the severed nonligated main branches of the main hepatic artery and the portal vein at the hilum immediately for preservation. After all the intrahepatic liquid media has drained to a large extent, the weight of the resected right liver lobe during the period of cold ischemia was determined with a calibrated electronic laboratory scale.
It is widely accepted that studies using preoperative and intraoperative liver volumetric measurements are based on the assumption that the density value is on the order of 1.00 g/mL, to facilitate the conversion of volumetric values to weight values.
Frequency (number) and relative frequency (percentage) for categorical data, mean (±standard deviation [SD]), and (minimum, maximum) values for normal data were summarized.
The difference between preoperative volumetry and real graft weight was graded into minimal difference (≤15%) and big difference (>15%). The correlation coefficient and degree of difference between the 2 volumetry programs were also determined. Linear regressions were calculated and the results were depicted as scatterplots. The test was said to be significant if the P-value is <5% level of significance. Data processing and data analysis were performed using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA).
Patient demographics and anthropometric data were retrieved from the records of all 215 donors and summarized as shown in Table 2. The majority of donors were male in both groups (69% and 59%, for Rapidia and Dr. Liver groups, respectively).
Approximately the mean ± SD ages of patients were 32.3 ± 11.0 years for those in the Rapidia group and 35.4 ± 11.8 years in Dr. Liver group. There was marginal evidence to suggest that the mean ages of patients between groups were different (P = 0.048).
There was no sufficient evidence that the mean ± SD body mass indices between groups (23.8 ± 3.2 kg/m2 for Rapidia vs. 23.2 ± 3.2 kg/m2 for Dr. Liver) were significantly different (P = 0.169). Similarly, there were no statistically significant evidences that the distributions of patients between groups were significantly different in terms of gender (P = 0.130), weight (P = 0.187), height (P = 0.420), body surface area (BSA) (P = 0.205), liver volume (P = 0.204), graft weight (P = 0.268), GRWR using graft volume (P = 0.492), GRWR using graft weight (P = 0.609) and presence of greater than 10% macrovesicular steatosis on liver biopsy (P = 0.498).
After collection of data from preoperative volumetry in both groups and real graft weights, we analyzed the degree of difference between 2 variables. Majority of cases in both groups overestimated the real graft weight. Underestimation was seen in 19 cases from the Rapidia group, and 17 cases from the Dr. Liver group. Absolute values of differences between preoperative volumetry and real graft weight were summarized and graded into minimal difference (≤15%) and big difference (>15%) as shown in Table 3.
Minimal difference (≤15%) from real graft weight was observed better with the Dr. Liver group (69.4% of cases). Big difference (>15%) from real graft weight was seen in almost half of the cases from the Rapidia group (44.9% of cases). Therefore, Dr. Liver software significantly showed better approximation of the right liver volume than Rapidia software when the real graft volume was used as standard (P = 0.035).
In the linear regression analysis, we found statistically significant positive correlation in both groups (P < 0.01). However, the correlation coefficient was better for the Dr. Liver group (R2 = 0.719), than for the Rapidia group (R2 = 0.688). The scatterplot diagram is shown in Fig. 1.
In Korea, LDLT has emerged as the dominant option due to the lack of liver grafts from deceased donors. Optimal graft size is an important element of both the donor evaluation and the excellent outcome of LDLT. Therefore, it is necessary to have a reliable preoperative estimation of appropriate graft size that will meet the metabolic demands of the recipient and at the same time provide an adequate liver remnant volume for donor safety.
Different formulas using patient's age, height, sex, body weight, BSA, and even maximal portal vein diameters have been used worldwide to estimate standard liver volume and have been published in literature [20]. The standard liver volume is used to predict the adequate liver graft volume required to prevent SFSS. Currently, the estimation of graft and remnant liver volume relies on noninvasive imaging such as CT scan which also provides detailed knowledge of liver anatomy that is essential for surgical planning.
Due to advances in technology, several generic manual or computer-aided protocols and commercially available stand-alone specialized virtual software systems have recently been advocated to simplify the volumetry calculation and hasten its process. However, each software has its own strengths and weaknesses.
Our study showed that Dr. Liver software significantly calculated right liver volumes better than Rapidia software with 69.4% of cases having less than 15% difference from real graft weight. This result was better than the study reported by Sakamoto et al. [21] which found a 32% underestimation to a 21% overestimation of the actual graft weight by volume.
Our linear regression analysis revealed a significantly positive correlation between estimated liver volume using Dr. Liver software and real graft weight. Furthermore, the correlation coefficient was better for the Dr. Liver group than for the Rapidia group. Although we did not do an analysis of time duration needed for volumetry and surgical planning, we observed that it took only 10–15 minutes to use the Dr. Liver software. Our results were comparable to those reported in literature by other authors [102223].
The software developers of Dr. Liver initiated a comparative study with another semi-automated program by using manual volumetry as standard. Their series showed that Dr. Liver was better than OsiriX (Freeware; Pixmeo, Bernex, Switzerland) in terms of mean difference in liver volume and processing time [24].
Age can be a factor for volume overestimation especially in young donors less than 30 years old as reported by previous studies [2526]. Recently, the donor selection criteria have been extended to include those in their 40's and 50's (older age group), therefore, our data showed significant difference in donor age between Rapidia group (earlier period) and Dr. Liver group (more recent period). However, the difference is minimal (32 years vs. 35 years in mean age) and most of the donors were in the young age range in both groups. Furthermore, contrary to the previous studies that used UW solution (Belzer UW, Bridge to Life Ltd., Columbia, SC, USA), we used HTK solution as the organ perfusion solution during flushing to prevent tissue dehydration. Kayashima et al. [26] suggested that younger donors' livers may have a more developed capillary vascular bed than older donors, which could be susceptible to dehydration by UW solution. However, Custodiol has a lower osmolarity (310 mOsm/L vs. 320 mOsm/L), lower sodium, and lower potassium content than the UW solution. Therefore, there will be less influence by donor age when HTK solution is used. Donor age difference between 2 groups did not confound the result.
In general, the accuracy of generic semimanual liver volumetry software such as Rapidia might be complicated by the degree of parenchymal fatty change. This is due to the fact that Rapidia is dependent on volume rendering techniques. However, we already adjusted the thresholding levels to include most of the liver parenchyma in cases of some fatty changes in the Rapidia group. Patients with severe fatty liver had been excluded from the study during donor evaluation [27]. In addition, the number of donors in the Rapidia group with more than 10% macrovesicular steatosis on liver biopsy was not statistically significant (P = 0.498) and 55.1% in this group had less than 15% difference from the actual graft weight. Furthermore, the way of manual setting of liver border can vary among operators, which may result in volume difference. Therefore, volume estimation using Dr. Liver is better since it does not require these adjustments (which can also be affected by intraobserver variability) and produced better correlation with actual graft weight. The difference in extraction process for fatty liver in both Rapidia and Dr. Liver softwares is illustrated in Fig. 2.
Dr. Liver software allows extraction of blood vessels and biliary structures, as previously described, then automatically excludes them from the volume calculation, thus generating a blood-free liver volume and minimizing discrepancy from actual graft weight observed in previous studies [1528]. This also eliminates the need to multiply the volume calculated by Dr. Liver with different formulas and conversion factors proposed by other authors to minimize discrepancy [29].
In conclusion, liver volumetry performed with the semiautomatic Dr. Liver software can accurately predict right graft size better than the semimanual Rapidia software. It can generate excellent volumetry results faster and facilitate preoperative planning in LDLT.
Figures and Tables
Fig. 1
Correlation between preoperative and real graft weight using the 2 programs. Scatterplot diagrams show a positive linear correlation for preoperative volume (Volumetry) and real graft weight (Graft Weight) determinations in Rapidia (A) and Dr. Liver (B). The correlation coefficient was better in the Dr. Liver group (R2 = 0.688 vs. R2 = 0.719). Rapidia software (Infinitt Co., Ltd., Seoul, Korea); Dr. Liver software (Virtual Liver Surgery Planning System, Humanopia Co. Ltd, Pohang, Korea).

Fig. 2
Difference in liver extraction process between Rapidia and Dr. Liver for normal and fatty liver. (A, B) Parenchyma of normal and fatty liver at same threshold range 83–150 (Rapidia). (C) Fatty liver at an adjusted threshold range 50–1,000 (Rapidia) to include most of parenchyma. (D, E) Parenchyma of normal and fatty liver (Dr. Liver) not dependent on attenuation/thresholding adjustments. Rapidia software (Infinitt Co., Ltd., Seoul, Korea); Dr. Liver software (Virtual Liver Surgery Planning System, Humanopia Co. Ltd, Pohang, Korea).
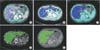
Table 2
Baseline characteristics of living donors

Values are presented as number, mean ± standard deviation, or number (%).
GRWR, graft-to-recipient weight ratio.
a)Graft volume by volumetry/recipient body weight. b)Graft weight measured intraoperatively/recipient body weight.
Rapidia software (Infinitt Co., Ltd., Seoul, Korea); Dr. Liver software (Virtual Liver Surgery Planning System, Humanopia Co. Ltd, Pohang, Korea).
References
1. Hashikura Y, Makuuchi M, Kawasaki S, Matsunami H, Ikegami T, Nakazawa Y, et al. Successful living-related partial liver transplantation to an adult patient. Lancet. 1994; 343:1233–1234.
2. Kawasaki S, Makuuchi M, Matsunami H, Hashikura Y, Ikegami T, Nakazawa Y, et al. Living related liver transplantation in adults. Ann Surg. 1998; 227:269–274.
3. Tanaka K, Uemoto S, Tokunaga Y, Fujita S, Sano K, Nishizawa T, et al. Surgical techniques and innovations in living related liver transplantation. Ann Surg. 1993; 217:82–91.
4. Lee SG, Park KM, Lee YJ, Hwang S, Choi DR, Ahn CS, et al. 157 adult-to-adult living donor liver transplantation. Transplant Proc. 2001; 33:1323–1325.
5. Chen CL, Fan ST, Lee SG, Makuuchi M, Tanaka K. Living-donor liver transplantation: 12 years of experience in Asia. Transplantation. 2003; 75:3 Suppl. S6–S11.
6. Makuuchi M, Sugawara Y. Technical progress in living donor liver transplantation for adults. HPB (Oxford). 2004; 6:95–98.
7. Lo CM, Fan ST, Liu CL, Wei WI, Lo RJ, Lai CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg. 1997; 226:261–269.
8. Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005; 5:2605–2610.
9. Nakayama Y, Li Q, Katsuragawa S, Ikeda R, Hiai Y, Awai K, et al. Automated hepatic volumetry for living related liver transplantation at multisection CT. Radiology. 2006; 240:743–748.
10. Suzuki K, Epstein ML, Kohlbrenner R, Garg S, Hori M, Oto A, et al. Quantitative radiology: automated CT liver volumetry compared with interactive volumetry and manual volumetry. AJR Am J Roentgenol. 2011; 197:W706–W712.
11. Bae KT, Giger ML, Chen CT, Kahn CE Jr. Automatic segmentation of liver structure in CT images. Med Phys. 1993; 20:71–78.
12. Gao L, Heath DG, Kuszyk BS, Fishman EK. Automatic liver segmentation technique for three-dimensional visualization of CT data. Radiology. 1996; 201:359–364.
13. Hermoye L, Laamari-Azjal I, Cao Z, Annet L, Lerut J, Dawant BM, et al. Liver segmentation in living liver transplant donors: comparison of semiautomatic and manual methods. Radiology. 2005; 234:171–178.
14. Okada T, Shimada R, Hori M, Nakamoto M, Chen YW, Nakamura H, et al. Automated segmentation of the liver from 3D CT images using probabilistic atlas and multilevel statistical shape model. Acad Radiol. 2008; 15:1390–1403.
15. Kim KW, Lee J, Lee H, Jeong WK, Won HJ, Shin YM, et al. Right lobe estimated blood-free weight for living donor liver transplantation: accuracy of automated blood-free CT volumetry-- preliminary results. Radiology. 2010; 256:433–440.
16. Karlo C, Reiner CS, Stolzmann P, Breitenstein S, Marincek B, Weishaupt D, et al. CT- and MRI-based volumetry of resected liver specimen: comparison to intraoperative volume and weight measurements and calculation of conversion factors. Eur J Radiol. 2010; 75(1):e107–e111.
17. Jung EJ, Ryu CG, Kim G, Kim SR, Park HS, Kim YJ, et al. Splenomegaly during oxaliplatin-based chemotherapy for colorectal carcinoma. Anticancer Res. 2012; 32:3357–3362.
18. Perandini S, Faccioli N, Zaccarella A, Re T, Mucelli RP. The diagnostic contribution of CT volumetric rendering techniques in routine practice. Indian J Radiol Imaging. 2010; 20:92–97.
19. Yang X, Yu HC, Choi Y, Lee W, Wang B, Yang J, et al. A hybrid semi-automatic method for liver segmentation based on level-set methods using multiple seed points. Comput Methods Programs Biomed. 2014; 113:69–79.
20. Pomposelli JJ, Tongyoo A, Wald C, Pomfret EA. Variability of standard liver volume estimation versus software-assisted total liver volume measurement. Liver Transpl. 2012; 18:1083–1092.
21. Sakamoto S, Uemoto S, Uryuhara K, Kim Id, Kiuchi T, Egawa H, et al. Graft size assessment and analysis of donors for living donor liver transplantation using right lobe. Transplantation. 2001; 71:1407–1413.
22. Mokry T, Bellemann N, Muller D, Lorenzo Bermejo J, Klauß M, Stampfl U, et al. Accuracy of estimation of graft size for living-related liver transplantation: first results of a semi-automated interactive software for CT-volumetry. PLoS One. 2014; 9:e110201.
23. Luciani A, Rusko L, Baranes L, Pichon E, Loze B, Deux JF, et al. Automated liver volumetry in orthotopic liver transplantation using multiphase acquisitions on MDCT. AJR Am J Roentgenol. 2012; 198:W568–W574.
24. Yang X, Lee W, Choi Y, You H. Development of a user-centered virtual liver surgery planning system. In : Proceedings of the 56th Human Factors and Ergonomics Society Annual Meeting; 2012 Oct 22-26; Boston (MA), USA. Santa Monica (CA): Human Factors and Ergonomics Society;2012. p. 772–776.
25. Yonemura Y, Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Gion T, et al. Validity of preoperative volumetric analysis of congestion volume in living donor liver transplantation using three-dimensional computed tomography. Liver Transpl. 2005; 11:1556–1562.
26. Kayashima H, Taketomi A, Yonemura Y, Ijichi H, Harada N, Yoshizumi T, et al. Accuracy of an age-adjusted formula in assessing the graft volume in living donor liver transplantation. Liver Transpl. 2008; 14:1366–1371.
27. Choi Y, Lee JM, Yi NJ, Kim H, Park MS, Hong G, et al. Heterogeneous living donor hepatic fat distribution on MRI chemical shift imaging. Ann Surg Treat Res. 2015; 89:37–42.
28. D'Onofrio M, De Robertis R, Demozzi E, Crosara S, Canestrini S, Pozzi Mucelli R. Liver volumetry: is imaging reliable? Personal experience and review of the literature. World J Radiol. 2014; 6:62–71.
29. Yoneyama T, Asonuma K, Okajima H, Lee KJ, Yamamoto H, Takeichi T, et al. Coefficient factor for graft weight estimation from preoperative computed tomography volumetry in living donor liver transplantation. Liver Transpl. 2011; 17:369–372.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download