This article has been corrected. See "Affiliation and corresponding author correction: Laparoscopic resection of retroperitoneal benign neurilemmoma" in Volume 92 on page 388.
Abstract
Purpose
The aim of this study was to verify that laparoscopic resection for treating retroperitoneal benign neurilemmoma (NL) is expected to be favorable for complete resection of tumor with technical feasibility and safety.
Methods
We retrospectively analyzed 47 operations for retroperitoneal neurogenic tumor at Yonsei University College of Medicine, Severance Hospital and Gangnam Severance Hospital between January 2005 and September 2015. After excluding 21 patients, the remaining 26 were divided into 2 groups: those who underwent open surgery (OS) and those who underwent laparoscopic surgery (LS). We compared clinicopathological features between the 2 groups.
Results
There was no significant difference in operation time, estimated blood loss, transfusion, complication, recurrence, or follow-up period between 2 groups. Postoperative hospital stay was significantly shorter in the LS group versus the OS group (OS vs. LS, 7.00 ± 3.43 days vs. 4.50 ± 2.16 days; P = 0.031).
Neurilemmoma (NL) is a Schwann cell tumor arising from the nerve sheath [1]. Benign NL occurs predominantly in the cranial and peripheral nerves of the upper limbs. Although rarely found in the retroperitoneal area (0.3%–3.2%) [2], NL is the most common benign soft tissue tumor in the retroperitoneum and constitutes approximately 1%–5% of all retroperitoneal tumors [34]. Retroperitoneal neurilemmoma (RNL) is usually discovered during routine medical check-ups or incidentally diagnosed in patients being treated for unrelated symptoms. They are usually solitary, and multiple or plexiform lesions are commonly associated with neurofibromatosis I or schwannomatosis (Fig. 1, 2) [5].
The treatment of choice for NL is surgical resection. Chemotherapy is generally not used. In some cases, radiosurgery or stereotactic radiotherapy may be effectively used to control further tumor growth. However, as NLs usually present as fusiform, round, or oval masses and are sharply circumscribed and encapsulated, surgical resection is the typical treatment. Because of difficulty approaching the retroperitoneal space and the surrounding internal organs (pancreas and duodenum) and major vessels, an open approach is generally preferred. As laparoscopic surgery (LS) has become more popular for the management of patients with abdominal diseases, interest in laparoscopic approaches to RNL is increasing [6].
LS was first introduced for the treatment of cholecystectomy in 1987. The indications for LS have expanded gradually from benign to malignant abdominal diseases [78]. But LS has some limitations, such as 2-dimensional vision and restricted range of motion. This can make surgeries more difficult to perform in patients who have retroperitoneal tumors, especially near the great vessels [9]. Open conversion is unavoidable if there is uncontrollable bleeding due to great vessel injury. However, with advances in laparoscopic surgical techniques and instruments, LS is being practiced more frequently than ever before (Fig. 3) [10].
We previously reported a case of RNL located between the inferior vena cava and aorta, which was successfully resected by a laparoscopic approach, suggesting that laparoscopic resection of even retroperitoneal pathologies can be feasible and safe [11]. Recently, several laparoscopic approaches to RNL have been reported [1213], but only as case reports. Herein, we report our accumulating experience with laparoscopic and open resections of RNL. The aim of this study was to evaluate the technical feasibility and safety of laparoscopic resection for treating RNL compared with open resection techniques. To the best of our knowledge, this study is one of the largest describing experiences at a single institution.
This study was approved by the Yonsei University Institutional Review Board (approval number: 4-2016-0758). We retrospectively analyzed 47 operations for retroperitoneal neurogenic tumors at Yonsei University College of Medicine, Severance Hospital and Gangnam Severance Hospital between January 2005 and September 2015. Twenty-one cases met the exclusion criteria. Eighteen cases were excluded owing to the diagnosis of other neurogenic tumors confirmed histopathologically after operation. Eleven patients were diagnosed with paraganglioma, and seven patients with ganglioneuroma. Furthermore, 3 cases were excluded owing to multiple RNL.
In all cases, CT scans were routinely performed before operation, and all pathology-based diagnoses were confirmed histopathologically after operation. Surgical approach method was determined according to each surgeon's preference. A total of 26 patients were divided into 2 groups according to the operation method: those who underwent open surgery (OS) (n = 10) and those who underwent LS (n = 16). Complete tumor removal with clear margin was performed in all cases. We compared the clinicopathological features, such as age, sex, clinical presentation, tumor size, tumor location, operation time, estimated blood loss, transfusion, postoperative hospital stay, complication, recurrence, and follow-up period. Also, we divided the 26 patients into 2 groups according to the operation period: those who underwent operation between 2005 and 2009 (early surgery group) (n = 14) and those who underwent operation between 2010 and 2015 (recent surgery group) (n = 12). We compared the clinicopathological features, such as age, sex, clinical presentation, tumor size, operation time, estimated blood loss, transfusion, postoperative hospital stay, and complication.
Continuous variables were described by means ± standard deviation, and categorical variables by frequency (%). The Pearson chi-square test or Student t-test was used to compare clinicopathological features in OS and LS groups for statistical assessments of associations. Statistical analysis was performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA) and differences were considered significant when P-values were less than 0.05.
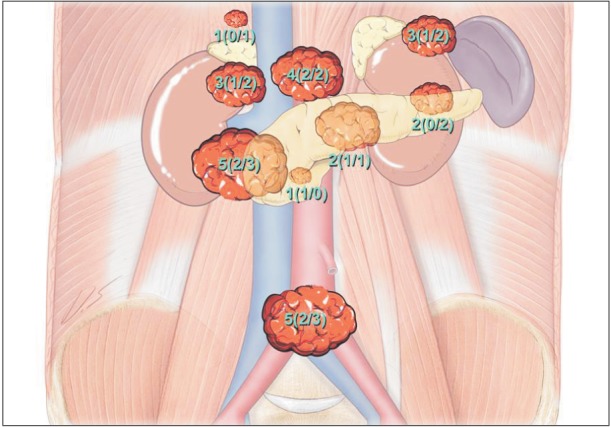
During the study period, 26 patients underwent operation for RNL. General characteristics of patients with resection of RNL are shown in Table 1. In most patients, tumors were identified incidentally during investigations for unrelated symptoms (n = 21, 80.8%). Only 5 patients had specific symptoms. Three patients presented with abdominal discomfort and 2 patients presented with back pain. Sixteen patients underwent LS and 10 patients underwent OS. All 16 patients who underwent LS were completed laparoscopically without open conversion. The mean follow-up period in the surveillance group was 15.5 ± 16.89 months. All of the patients are alive and disease-free. Tumor locations and frequencies are shown in Fig. 2. Frequent origin sites for RNL were between the inferior vena cava and pancreas head (OS vs. LS, n = 2 vs. n = 3) and pelvis (OS vs. LS, n = 2 vs. n = 3). Other RNL were located on the inferior vena cava (OS vs. LS, n = 1 vs. n = 2), left renal vein (OS vs. LS, n = 1 vs. n = 1), pancreas tail (OS vs. LS, n = 0 vs. n = 2), celiac trunk (OS vs. LS, n = 2 vs. n = 2), superior mesentery artery (OS vs. LS, n = 1 vs. n = 0), right para-aortic area (OS vs. LS, n = 0 vs. n = 1), and between the spleen and left kidney (OS vs. LS, n = 1 vs. n = 2). There were no differences in distribution of tumor origin site between the OS and LS groups (Fig. 4).
We compared our LS group results to those of conventional OS group. There were no significant differences in preoperative demographics and clinical data between the 2 groups for age, sex, presentation, or tumor size (Table 2). There were no significant differences between the 2 groups for operation time, estimated blood loss, transfusion, complication, recurrence, or follow-up period. However, postoperative hospital stay was significantly shorter in the LS group versus the OS group (OS vs. LS, 7.00 ± 3.43 days vs. 4.50 ± 2.16 days; P= 0.031) (Table 3).
During the 10-year study period, there were no significant characteristic differences in terms of chronological change in clinical practice of RNL (Table 4). The early surgery group (2005–2009) and recent surgery group (2010–2015) had similar characteristics, such as age, tumor size, and postoperative hospital stay. Also, sex distribution and manifestations between the 2 groups were similar. Although the recent surgery group had shorter operation times and less blood loss, the difference was not significant (P = 0.273, P = 0.211).
Due to recent developments in laparoscopic surgical experience, techniques, and instruments, LS is frequently conducted to treat retroperitoneal space disease [14]. At our institution, we performed 16 cases of LS for the treatment of RNL over a 10-year period. According to a literature review of cases reporting RNL, our patients' characteristics were similar to those of other institutions [215]. We performed LS more frequently than other institutions due to institutional preferences and experience with laparoscopic approaches.
Patients in both OS and LS groups had comparable preoperative demographic and clinical preoperative characteristics. In this study, the LS group experienced shorter postoperative hospital stays compared to the OS group, indicating smoother recovery. Furthermore, no patients in the LS group required transfusions, while 2 patients in the OS group received transfusions. Two complications were detected in the LS group, but they were minor complications such as lymphocele and chyle leakage. We observed no cases of recurrence among our patients. As aforementioned, in 2 groups with similar demographics, the laparoscopic approach is shown to be not inferior to OS for surgical outcomes. Previously known advantages of minimally invasive surgery such as shorter hospital stay and less postoperative scarring were also shown in this study.
Because of the possibility of local recurrence and malignant changes in benign NL, complete resection of RNL is considered to be very important. In the past, some authors had claimed that laparoscopic resection of RNL is unreliable. Because RNLs tend to be in close proximity to major vessels, surgery can result in uncontrollable bleeding. Tumor proximity to the ureter or kidney, as well unfamiliar anatomy, limited range of motion, and lack surgeon experience are also complicating factors [16]. However, over time, many studies have suggested that laparoscopic resection of NL may be performed more completely and safely than under open contexts [17]. Similar to previous studies, our results suggest that laparoscopic resection of RNL is a safe and feasible technique.
The limitations of this study should be noted. First, the retrospective nature of the study made it impossible to completely avoid selection biases. Furthermore, selection bias is inevitable due to the fact that 4 different surgeons were involved in the study and performed surgeries with their own surgical preferences. Surgeons A and C carried out laparoscopic surgeries (surgeon A, 10 cases; surgeon C, 1 case), whereas surgeon B performed both open and laparoscopic surgeries (4 cases [OS], 5 cases [LS], respectively), and surgeon D carried out 6 open surgeries. A randomized, prospective study in a sample that is stratified and matched for age, tumor location, tumor size, and medical comorbidities would be ideal for validating the feasibility of laparoscopic resection of RNL. Second, our study sample was small. Collection of RNL cases by literature review may be helpful to address this limitation.
In conclusion, laparoscopic resection has similar clinical outcomes and shorter postoperative hospital stays compared to open resection when used to treat RNL. Considering that most RNLs show benign clinical courses, we suggest that laparoscopic resection of RNL is feasible and safe when conducted with appropriate laparoscopic technique and proper patient selection.
ACKNOWLEDGEMENTS
The authors would like to thank Dong-Su Jang, MFA, (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the illustrations.
References
1. DasGupta TK, Brasfield RD, Strong EW, Hajdu SI. Benign solitary Schwannomas (neurilemomas). Cancer. 1969; 24:355–366. PMID: 5796779.

2. Mrugala MM, Batchelor TT, Plotkin SR. Peripheral and cranial nerve sheath tumors. Curr Opin Neurol. 2005; 18:604–610. PMID: 16155448.

3. Nah YW, Suh JH, Choi DH, Ko BK, Nam CW, Kim GY, et al. Benign retroperitoneal schwannoma: surgical consideration. Hepatogastroenterology. 2005; 52:1681–1684. PMID: 16334756.
4. Kapila K, Mathur S, Verma K. Schwannomas: a pitfall in the diagnosis of pleomorphic adenomas on fine-needle aspiration cytology. Diagn Cytopathol. 2002; 27:53–59. PMID: 12112817.

5. Pinson CW, ReMine SG, Fletcher WS, Braasch JW. Long-term results with primary retroperitoneal tumors. Arch Surg. 1989; 124:1168–1173. PMID: 2802979.

6. Petrucciani N, Sirimarco D, Magistri P, Antolino L, Gasparrini M, Ramacciato G. Retroperitoneal schwannomas: advantages of laparoscopic resection. Review of the literature and case presentation of a large paracaval benign schwannoma (with video). Asian J Endosc Surg. 2015; 8:78–82. PMID: 25598061.

7. Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994; 4:146–148. PMID: 8180768.
8. Kakisako K, Sato K, Adachi Y, Shiraishi N, Miyahara M, Kitano S. Laparoscopic colectomy for Dukes A colon cancer. Surg Laparosc Endosc Percutan Tech. 2000; 10:66–70. PMID: 10789575.

9. Ploussard G, Xylinas E, Paul A, Gillion N, Salomon L, Allory Y, et al. Is robot assistance affecting operating room time compared with pure retroperitoneal laparoscopic radical prostatectomy? J Endourol. 2009; 23:939–943. PMID: 19473064.

10. Cadeddu MO, Mamazza J, Schlachta CM, Seshadri PA, Poulin EC. Laparoscopic excision of retroperitoneal tumors: technique and review of the laparoscopic experience. Surg Laparosc Endosc Percutan Tech. 2001; 11:144–147. PMID: 11330383.
11. Kang CM, Kim DH, Lee WJ. Ten years of experience with resection of left-sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surg Endosc. 2010; 24:1533–1541. PMID: 20054579.

12. Okuyama T, Tagaya N, Saito K, Takahashi S, Shibusawa H, Oya M. Laparoscopic resection of a retroperitoneal pelvic schwannoma. J Surg Case Rep. 2014; (1):DOI: 10.1093/jscr/rjt122.

13. Pazouki A, Khalaj A, Shapoori P, Vaziri M, Najafi L. Laparoscopic resection of a retroperitoneal schwannoma. Surg Laparosc Endosc Percutan Tech. 2011; 21:e326–e328. PMID: 22146184.

14. Choi KS, Chung JC, Kim HC. Feasibility and outcomes of laparoscopic enucleation for pancreatic neoplasms. Ann Surg Treat Res. 2014; 87:285–289. PMID: 25485235.

15. Daneshmand S, Youssefzadeh D, Chamie K, Boswell W, Wu N, Stein JP, et al. Benign retroperitoneal schwannoma: a case series and review of the literature. Urology. 2003; 62:993–997. PMID: 14665342.

16. Foote MN, Luongo V, Marino ER. Benign giant retroperitoneal neurilemmoma. Ann Surg. 1963; 157:719–724. PMID: 13958661.

17. Yoshino T, Yoneda K. Laparoscopic resection of a retroperitoneal ancient schwannoma: a case report and review of the literature. Anticancer Res. 2008; 28(5B):2889–2891. PMID: 19031930.
Fig. 1
Histologic findings of retroperitoneal neurilemmoma. (A) Retroperitoneal neurilemmoma cell had spindle shape and wavy nucleus without atypia (H&E, ×100), In immunohistocheminal stain, (B) S-100 (positive, ×200), (C) Smooth muscle actin (negative, ×200), (D) CD34 (negative, ×200) were verified.
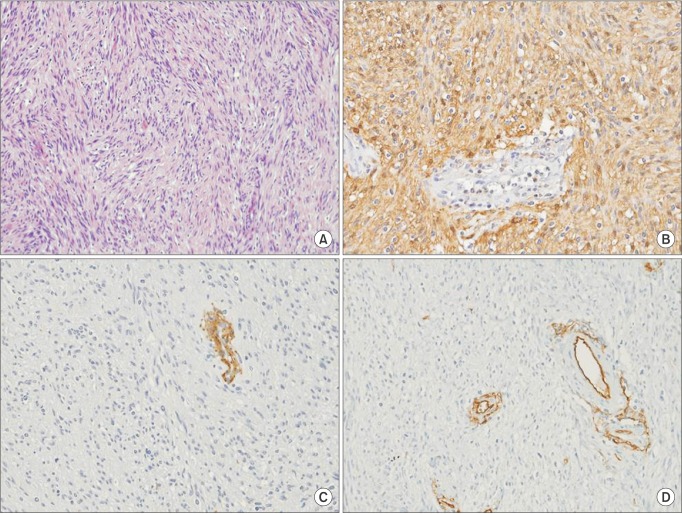
Fig. 2
Preoperative image modality findings and gross appearance of retroperitoneal neurilemmoma. CT scan (A) and MRI (B) reveal 3-cm-sized encapsulated oval mass, and fluorodeoxyglucose scan (C) reveals the hypermetabolic nature of the tumor. (D) Tumor has well-circumscribed margin.
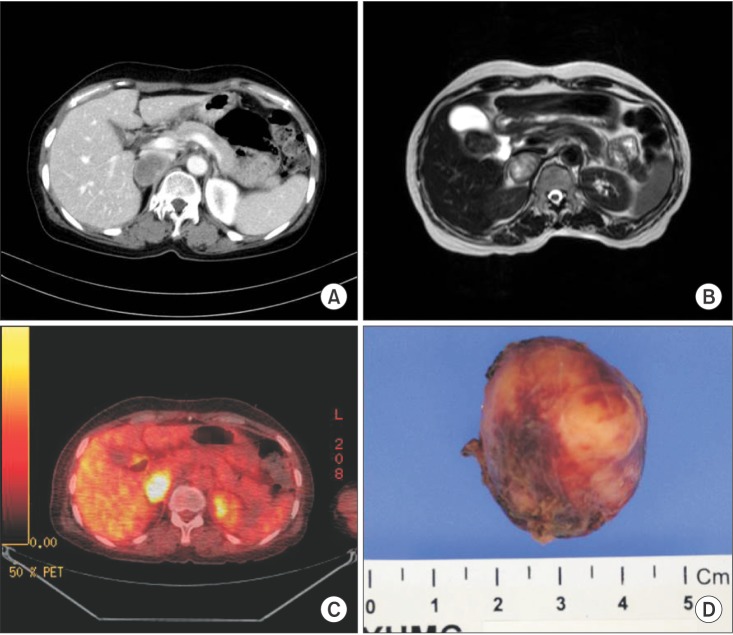
Fig. 3
Laparoscopic surgery of retroperitoneal neurilemmoma. (A) The tumor was located between right adrenal gland and IVC and (B) compressing the IVC to the anterior portion. (C) Cord-like structure was resected by surgical stapler. (D) The postoperative patient view. Right upper quadrant port site was used for drain placement. RNL, retroperitoneal neurilemmoma; CBD, common bile duct; IVC, inferior vena cava; Cd, cord-like structure.
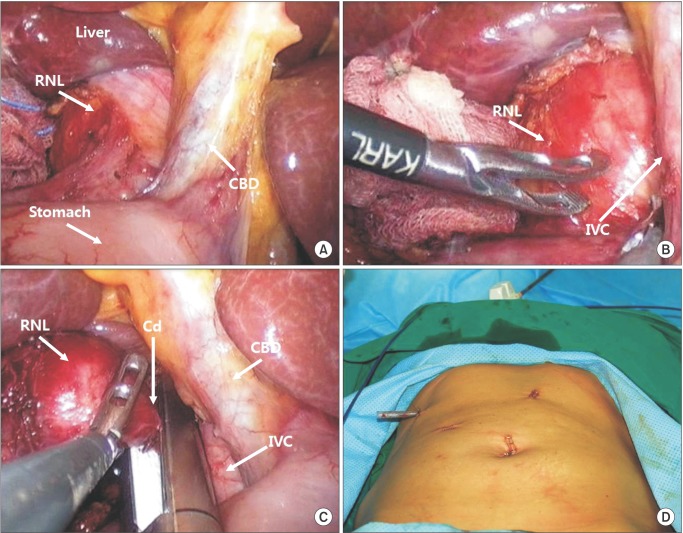
Fig. 4
Location of retroperitoneal neurilemmoma resected by open and laparoscopic surgery. Numbers are presented as total number (open surgery/laparoscopic surgery).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download