Abstract
Ovarian cancer is the seventh most common cancer in the world and the fifth most common cause of death from cancer; it is responsible for over half of all deaths related to gynecological cancers. The presence of lymphatic metastasis is an important prognostic factor in ovarian cancer. Nodal metastases to the pelvic and the para-aortic lymph nodes are common, particularly in an advanced of the disease (stages III-IV). The finding of distant nodal metastasis, especially subclavian lymph node metastasis, from ovarian carcinoma is very uncommon. 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) or FDG-PET/computed tomography (CT) provides an improved imaging for detecting metastatic lymph nodes in patients with ovarian cancer. Immunohistochemically, ovarian carcinoma cells are positive for estrogen receptor, progesterone receptor, cancer antigen 125, Wilms' tumor 1 protein, and p53; they are negative for thyroid transcription factor (TTF-1) and caudal-related homeobox 2 (CDX-2). This report describes a Korean woman diagnosed with ovarian cancer with subclavian lymph node metastasis revealed by FDG PET/CT and verified by an immunohistochemical staining. Differentiating between the primary ovarian lesion and the metastatic lesion will allow the initiation of an appropriate treatment and help predict the prognosis.
Ovarian cancer is the fifth most common cause of death from cancer and represents over half of all deaths related to gynecological cancers.1,2 Epithelial ovarian cancer classically spreads by exfoliation of cancerous cells into the intra-abdominal cavity, movement of the cells through lymphatic channels, and hematogenous dissemination. Lymph nodes are involved in about 50-70% of cases of advanced ovarian cancer.3,4 The presence of lymphatic metastasis is an important prognostic factor in ovarian cancer.3,5,6,7 Nodal metastases to the pelvic and para-aortic lymph nodes are common, particularly in advanced disease (stages III-IV).8
The finding of distant nodal metastasis is very uncommon. Moreover, subclavian lymph node metastases secondary to ovarian carcinoma are extremely rare and they are considered a poor prognostic sign. Positron emission tomography (PET) or PET/computed tomography (PET/CT) using 18F-fluorodeoxyglucose (FDG) performs superior imaging in the detection of lymph node metastasis in patients with ovarian cancer.9,10,11,12,13 Immunohistochemically, ovarian carcinoma cells are positive for estrogen receptor (ER), progesterone receptor (PR), cancer antigen 125 (CA-125), Wilms' tumor 1 protein (WT1), and p53. They are negative for thyroid transcription factor (TTF-1) and caudal-related homeobox 2 (CDX-2).
This report describes a Korean woman diagnosed with ovarian cancer in whom subclavian lymph node metastasis was revealed by FDG PET/CT and verified by immunohistochemical staining.
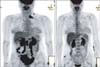
A 47-year-old Asian perimenopausal woman presented with abdominal pain and vomiting. Abdominal and pelvic computed tomography showed an ovarian tumor that was 15 cm in diameter. She had no relevant medical or surgical history. Her level of serum CA-125 was 971.3 U/mL (the normal range is 0 to 35 U/mL). Unexpectedly, the FDG-PET/CT scan showed hypermetabolic activity in the left subclavian lymph nodes (standardized uptake value [SUVmax] = 15.8) (Fig. 1). Before surgery, the physician decided to examine the subclavian lymph nodes. On physical examination, a mass was not palpable. Ultrasonography of the neck was performed, which showed multiple variably sized lymph nodes in the left subclavian fossa. Ultrasonography-guided biopsy was performed, and the pathology revealed metastatic carcinoma (Fig. 2). Immunohistochemical staining was positive for ER, PR, p53, p16, and WT1. However, staining for TTF-1 was negative.
The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, total omentectomy, pelvic lymphadenectomy, and para-aortic lymphadenectomy. During the surgery, a 10 cm cystic mass of the right ovary and a 4 cm sized solid mass of the left ovary were found infiltrating the posterior cul-de-sac. Furthermore, several masses with maximum diameters of 6 cm and 4 cm were found in the right and left para-aortic lymph nodes, respectively. The final diagnosis was stage IIIc serous adenocarcinoma. The pathology was positive for cancer in the pelvic and para-aortic lymph nodes (25/51). Subsequently, our patient underwent six cycles of paclitaxel (175 mg/m2) plus carboplatin at an area under the curve (AUC) of 5 mg given on day one of a 21-day cycle. The follow-up FDG-PET/CT at the end of chemotherapy showed no evidence of recurrence of residual lesions in the neck, pelvis, or elsewhere (Fig. 1). However, serum concentrations of CA-125 (36.57 U/mL) failed to decrease below the normal limit after six cycles; thus, the physician decided to administer an additional three cycles of paclitaxel plus carboplatin. After a total of nine cycles, the serum CA-125 levels decreased and entered the normal range (23.51 U/mL), and the follow-up FDG-PET/CT still showed no evidence of recurrence.
The most common routes for spread of ovarian cancer are lymphatic dissemination through the nearby internal organs. The most frequent sites of lymph node involvement are the abdominal (47%), para-aortic (38%), mediastinal (29%), and pelvic (17%) regions.14 Extra-abdominal metastasis is considered to be infrequent. Especially, metastases to subclavian lymph nodes has rarely been reported in ovarian cancer.
Several studies have shown that distant lymph node metastases in ovarian cancer can be detected by FDG-PET/CT and it was shown that preoperative FDG PET/CT can be a useful tool. But pathological confirmation of the PET/CT-positive lymph nodes was not performed.9,11,12 Although the histologic diagnosis of metastatic lesions can be difficult, immunohistochemistry has made the diagnosis of these lesions more accurate. The markers that helped to confirm the diagnosis were ER, PR, CA-125, ST1, and p53. Furthermore, the negative TTF-1 and CDX2 staining indicated that the carcinoma was not pulmonary or colorectal carcinoma. TTF-1 is a selective marker of adenocarcinoma of pulmonary origin in cytologic preparations.15 In our case, a perimenopausal woman was found to have subclavian lymph node metastases during the initial preoperative work-up for FDG-PET/CT. Subsequently, we concluded through immunohistochemical analysis that the metastases originated from the ovary.
About 25% of gynecological cancers including ovarian cancer will occur in premenopausal and perimenopausal women, and approximately 30% of ovarian neoplasms in postmenopausal women are malignant.16,17 In the advanced stage of epithelial ovarian cancer, preoperative FDG PET/CT and immunohistochemical analysis to confirm the presence of distant metastasis in lymph nodes are warranted in order to evaluate whether the lymph nodes should be taken into consideration when deciding on the extent of the treatment or when assessing the prognosis.
Differentiating between the primary ovarian lesion and the metastatic lesion allowed for the initiation of the appropriate treatment and helped determine the prognosis.
Figures and Tables
References
1. Bristow RE, Duska LR, Lambrou NC, Fishman EK, O'Neill MJ, Trimble EL, et al. A model for predicting surgical outcome in patients with advanced ovarian carcinoma using computed tomography. Cancer. 2000; 89:1532–1540.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.
3. Burghardt E, Girardi F, Lahousen M, Tamussino K, Stettner H. Patterns of pelvic and paraaortic lymph node involvement in ovarian cancer. Gynecol Oncol. 1991; 40:103–106.
4. Onda T, Yoshikawa H, Yokota H, Yasugi T, Taketani Y. Assessment of metastases to aortic and pelvic lymph nodes in epithelial ovarian carcinoma. A proposal for essential sites for lymph node biopsy. Cancer. 1996; 78:803–808.
5. Chen SS. Survival of ovarian carcinoma with or without lymph node metastasis. Gynecol Oncol. 1987; 27:368–372.
6. Scarabelli C, Gallo A, Zarrelli A, Visentin C, Campagnutta E. Systematic pelvic and para-aortic lymphadenectomy during cytoreductive surgery in advanced ovarian cancer: potential benefit on survival. Gynecol Oncol. 1995; 56:328–337.
7. Tsuruchi N, Kamura T, Tsukamoto N, Akazawa K, Saito T, Kaku T, et al. Relationship between paraaortic lymph node involvement and intraperitoneal spread in patients with ovarian cancer--a multivariate analysis. Gynecol Oncol. 1993; 49:51–55.
8. Parazzini F, Valsecchi G, Bolis G, Guarnerio P, Reina S, Polverino G, et al. Pelvic and paraortic lymph nodal status in advanced ovarian cancer and survival. Gynecol Oncol. 1999; 74:7–11.
9. Yuan Y, Gu ZX, Tao XF, Liu SY. Computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with ovarian cancer: a meta-analysis. Eur J Radiol. 2012; 81:1002–1006.
10. Castellucci P, Perrone AM, Picchio M, Ghi T, Farsad M, Nanni C, et al. Diagnostic accuracy of 18F-FDG PET/CT in characterizing ovarian lesions and staging ovarian cancer: correlation with transvaginal ultrasonography, computed tomography, and histology. Nucl Med Commun. 2007; 28:589–595.
11. Nam EJ, Yun MJ, Oh YT, Kim JW, Kim JH, Kim S, et al. Diagnosis and staging of primary ovarian cancer: correlation between PET/CT, Doppler US, and CT or MRI. Gynecol Oncol. 2010; 116:389–394.
12. Risum S, Høgdall C, Loft A, Berthelsen AK, Høgdall E, Nedergaard L, et al. Does the use of diagnostic PET/CT cause stage migration in patients with primary advanced ovarian cancer? Gynecol Oncol. 2010; 116:395–398.
13. Hynninen J, Auranen A, Carpén O, Dean K, Seppänen M, Kemppainen J, et al. FDG PET/CT in staging of advanced epithelial ovarian cancer: frequency of supradiaphragmatic lymph node metastasis challenges the traditional pattern of disease spread. Gynecol Oncol. 2012; 126:64–68.
14. Skagias L, Ntinis A, Vasou O, Kondi-Pafiti A, Politi E. Ovarian carcinoma presenting with axillary lymph node metastasis: a case diagnosed by fine-needle aspiration and brief review of the literature. Diagn Cytopathol. 2008; 36:891–893.
15. Hecht JL, Pinkus JL, Weinstein LJ, Pinkus GS. The value of thyroid transcription factor-1 in cytologic preparations as a marker for metastatic adenocarcinoma of lung origin. Am J Clin Pathol. 2001; 116:483–488.
16. Hershlag A, Schuster MW. Return of fertility after autologous stem cell transplantation. Fertil Steril. 2002; 77:419–421.
17. Kim TH, Lee HH, Chung SH, Kwak JJ, Lee BI, Hong YP. Serous adenocarcinoma arising from ovarian endometriosis after menopause. Korean J Obstet Gynecol. 2010; 53:365–370.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download