Abstract
Uterine papillary serous carcinoma (UPSC) is an aggressive form of endometrial cancer characterized by a high recurrence rate and poor prognosis. We report a case of a 58-year-old post-menopausal woman with an abdominal wall metastasis in stage IA UPSC. After surgical staging, she did not receive additional adjuvant therapy. An egg sized palpable mass developed in the right lower abdomen after 8 months. Both Abdominopelvic computed tomography (CT) and positron emission tomography (PET)-CT revealed a metastatic lesion in the abdominal wall. Hence, surgical excision was performed. The pathological findings showed metastatic UPSC with clear resection margin. After the diagnosis of UPSC metastasis in the abdominal wall, she received chemotherapy utilizing paclitaxel and carboplatin. After 3 years, no evidence of recurrence was found. Therefore, we suggest that even when UPSC is confined to the endometrium without lymph node metastasis and without lymphovascular invasion, chemotherapy should be considered as a postoperative adjuvant therapy.
Uterine Papillary Serous Carcinoma (UPSC) is an uncommon but aggressive type of endometrial carcinoma with high recurrence rate and poor survival outcomes. Although UPSC represents approximately 3% to 10% of the endometrial carcinomas, it accounts for almost 40% of all endometrial cancer-related death. The tumor often deeply invades the myometrium and has a propensity for peritoneal spread. Unfortunately, advanced-stage disease or recurrence is common even when UPSC is apparently only minimally invasive or even confined to the endometrium.1,2,3
We report a case of a 58-year-old patient with abdominal wall metastasis in UPSC stage IA: no lymphovascular invasion and no metastasis in regional lymph nodes were present.
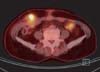
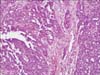
A 58-year-old post-menopausal woman presented UPSC defined through the International Federation of Obstetrics and Gynecology (FIGO) at stage IA, diagnosed in October 2008. Her past medical history had reported a modified radical mastectomy on right breast due to infiltrating ductal carcinoma in August 2001 and the patient subsequently took toremifene, 40 mg daily, for 5 years. She appeared healthy postoperatively. In October 2008, an abnormal hypermetabolic lesion on her uterus was noted on a follow-up positron emission tomography-computed tomography (PET-CT; GE Healthcare, Milwaukee, WI, USA). Her endometrial biopsy revealed papillary serous adenocarcinoma with negative estrogen receptor and progesterone receptor. She underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy with surgical staging in October 2008. The peritoneal cavity was entered through low midline skin incision and drain was inserted. The pathological finding revealed stage IA UPSC that the tumor was limited to the endometrium without lymphovascular space invasion and no lymph node metastasis (Fig. 1). She had not taken adjuvant therapy. After surgical staging, an egg sized palpable mass had developed in her lower right abdomen after 8 months. Abdominopelvic CT (showed a 4.4 × 4.1 cm sized low-attenuated septated cystic mass in the abdominal wall (Fig. 2). PET-CT revealed an abnormal hypermetabolic lesion in the abdominal wall with no other metastasis (Fig. 3). Surgical excision was performed. The frozen and permanent pathological findings showed metastatic UPSC with clear resection margin (Fig. 4). The wide defect of the abdominal fascia was closed with a synthetic mesh. After diagnosis of UPSC metastasis in the abdominal wall, she received chemotherapy utilizing paclitaxel (body surface area [BSA] × 175 mg) and carboplatin (area under the curve [AUC] × 5) every 3 weeks. 3 years have passed since and she is alive. The latest abdominopelvic CT showed no evidence of recurrent masses or distant metastases. Serum cancer antigen 125 (CA-125) was within the normal limit.
Even among diseases that occur after menopause, UPSC is a highly malignant variant of endometrial adenocarcinoma that histologically and clinically resembles ovarian papillary serous carcinoma. It is usually found in an advanced stage.4 Lymphovascular space invasion is common in the myometrium. The meticulous surgical staging is important for predicting the prognosis of UPSC, because most patients tend to be upstaged after a comprehensive surgical staging.1,4,5,6
UPSC truly confined to the uterus has an overall excellent prognosis, whereas patients with extrauterine disease, even if it is revealed only in a microscopy, almost always suffers from recurrence and subsequent death caused by the tumor.7,8
UPSC tends to spread in a fashion similar to ovarian cancer with a high propensity for upper abdominal relapse and has a poor prognosis even in the absence of deep myometrial invasion or lymph node metastasis. With such a poor survival rate, additional adjuvant therapy with whole abdomen radiation or systemic chemotherapy has been applied in hopes of improving survival. Lim et al.9 reported 78 patients with stage I-IIIA UPSC: 58 patients were treated with whole abdominal radiation and 20 patients did not receive treatment. The corresponding 5-year disease-specific survival rates were 74.9% and 41.3%, respectively (P = 0.04).9 In GOG study 94, the 3-year disease-free and overall survival rates for the 60 patients with stage III papillary serous or clear cell carcinomas were 40.9% and 45.0%, respectively.10 The data on whole abdominal radiation is somewhat encouraging, but effective systemic therapy is needed, because the rate of relapse is still substantial.
Trials investigating paclitaxel and carboplatin in UPSCs reported response rates of 60% to 70%.11,12 Gehrig13 reported that paclitaxel and platinum-based chemotherapy for three cycles followed by radiation therapy and then an additional three cycles of chemotherapy was tried for advanced-staged UPSC. The progression free survival in 9 patients was 46.4 months.13
A study of 74 patients with stage I UPSC between 1987 and 2004, who underwent complete staging at Yale University, reported that stage IA patients with residual uterine disease, who were treated with platinum-based chemotherapy, had no recurrence (n = 7), while 6 of 14 (43%) stage IA patients with residual uterine disease, who did not receive chemotherapy, experienced a recurrence. The 15 patients with stage IB UPSC who received platinum-based chemotherapy had no recurrences but 10 of the 13 (77%) stage IB patients who did not receive chemotherapy had recurrence. Platinum-based chemotherapy was associated with improved progression-free (P < 0.01) and overall survival (P < 0.05). In addition, no patient who received radiation to the vaginal cuff had recurrence at the cuff, but 6 of 31 (19%) patients who were not treated with vaginal radiation had recurrence at the cuff.14
These retrospective, limited data supported the potential benefit of platinum-based chemotherapy with vaginal cuff irradiation in UPSC. A randomized, prospective study is needed to define optimal treatment stage I UPSC.
In conclusion, we report a case of UPSC abdominal wall metastasis. Systemic chemotherapy was an important aspect of the adjuvant therapy in our UPSC study. We suggest that even when UPSC is confined to the endometrium without regional lymph node metastasis and with no lymphovascular invasion, chemotherapy should be considered as a postoperative adjuvant therapy.
Figures and Tables
Fig. 1
Papillary serous adenocarcinoma, a primary lesion in the uterus, shows no myometrial invasion (H&E × 400).
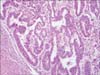
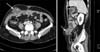
Fig. 2
Abdominopelvic computed tomography shows a 4.4 × 4.1 cm sized low attenuated septated cystic mass in the right lower quadrant abdominal wall (arrow).
References
1. Goff BA, Kato D, Schmidt RA, Ek M, Ferry JA, Muntz HG, et al. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol. 1994; 54:264–268.
2. Carcangiu ML, Chambers JT. Uterine papillary serous carcinoma: a study on 108 cases with emphasis on the prognostic significance of associated endometrioid carcinoma, absence of invasion, and concomitant ovarian carcinoma. Gynecol Oncol. 1992; 47:298–305.
3. Chan JK, Loizzi V, Youssef M, Osann K, Rutgers J, Vasilev SA, et al. Significance of comprehensive surgical staging in noninvasive papillary serous carcinoma of the endometrium. Gynecol Oncol. 2003; 90:181–185.
4. Wilson TO, Podratz KC, Gaffey TA, Malkasian GD Jr, O'Brien PC, Naessens JM. Evaluation of unfavorable histologic subtypes in endometrial adenocarcinoma. Am J Obstet Gynecol. 1990; 162:418–423. discussion 23-6.
5. Chambers JT, Merino M, Kohorn EI, Peschel RE, Schwartz PE. Uterine papillary serous carcinoma. Obstet Gynecol. 1987; 69:109–113.
6. Wheeler DT, Bell KA, Kurman RJ, Sherman ME. Minimal uterine serous carcinoma: diagnosis and clinicopathologic correlation. Am J Surg Pathol. 2000; 24:797–806.
7. Huh WK, Powell M, Leath CA 3rd, Straughn JM Jr, Cohn DE, Gold MA, et al. Uterine papillary serous carcinoma: comparisons of outcomes in surgical Stage I patients with and without adjuvant therapy. Gynecol Oncol. 2003; 91:470–475.
8. Havrilesky LJ, Secord AA, Bae-Jump V, Ayeni T, Calingaert B, Clarke-Pearson DL, et al. Outcomes in surgical stage I uterine papillary serous carcinoma. Gynecol Oncol. 2007; 105:677–682.
9. Lim P, Al Kushi A, Gilks B, Wong F, Aquino-Parsons C. Early stage uterine papillary serous carcinoma of the endometrium: effect of adjuvant whole abdominal radiotherapy and pathologic parameters on outcome. Cancer. 2001; 91:752–757.
10. Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley HD, Malfetano JH, et al. Whole abdominal radiotherapy in the adjuvant treatment of patients with stage III and IV endometrial cancer: a gynecologic oncology group study. Gynecol Oncol. 2005; 97:755–763.
11. Zanotti KM, Belinson JL, Kennedy AW, Webster KD, Markman M. The use of paclitaxel and platinum-based chemotherapy in uterine papillary serous carcinoma. Gynecol Oncol. 1999; 74:272–277.
12. Ramondetta L, Burke TW, Levenback C, Bevers M, Bodurka-Bevers D, Gershenson DM. Treatment of uterine papillary serous carcinoma with paclitaxel. Gynecol Oncol. 2001; 82:156–161.
13. Gehrig PA. Uterine papillary serous carcinoma: a review. Expert Opin Pharmacother. 2007; 8:809–816.
14. Kelly MG, O'Malley DM, Hui P, McAlpine J, Yu H, Rutherford TJ, et al. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol Oncol. 2005; 98:353–359.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download