Abstract
Objectives
Borderline ovarian tumors (BOT) are premalignant lesions. Approximately 10% of all epithelial ovarian cancers are known to be hereditary with hereditary breast and ovarian cancer (HBOC) accounting for approximately 90% of cases; the remaining 10% are attributable to Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC). The aim of our study is to estimate this risk based on screening immunohistochemistry (IHC).
Methods
Thirty-four patients diagnosed with BOT were identified. Family history, clinical characteristics, and IHC data (breast cancer 1, early onset [BRCA1], breast cancer 2, early onset [BRCA2], mutS homolog 2 [MSH2], mutL homolog 1 [MLH1]) were collected for all cases from the patients' medical charts. Nuclear staining of the tumor was scored as negative and positive.
Ovarian cancer is the eighth most common malignancy in women worldwide with a mortality rate of over 140,000 deaths per year.1 Borderline ovarian tumors (BOT) are recognized as a vague entity of ovarian tumors between benign and malignant tumors. BOTs are typically detected 20 years earlier than invasive ovarian carcinoma.2
The annual incidence of BOT is 1.5-2.5 per 100,000 and approximately 3,000 cases of BOT are diagnosed each year in the United States.3 Since the 1970s BOT has become more common among white women of reproductive age. BOT is classified into five categories among which the most common types are serous and mucinous. For the serous type, 70% of cases are stage I with a survival rate of almost 100% and 30% are advanced stage with a survival rate of 95.3%. For the mucinous type, 82% of cases are stage I with a survival rate of 99-100% and 18% are advanced stages with a survival rate of 50%.4 Some studies have reported that the recurrence rate for BOT ranges from 8% to 32%.5
Hereditary ovarian cancer represents approximately 10% of all epithelial ovarian cancers.6,7 The two most common hereditary cancer syndromes with regard to ovarian cancer are hereditary breast and ovarian cancer (HBOC) and Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC). HBOC accounts for approximately 90% of cases, and the remaining 10% are attributable to Lynch syndrome. The frequency of breast cancer 1, early onset (BRCA1) or breast cancer 2, early onset (BRCA2) mutations in the general population is estimated to be between 1 in 300 and 1 in 800.8 BRCA1/2 are located on chromosomes 17q21 (22 exons, 80 kb DNA) and 13q12-13 (26 exons, 70 kb DNA)9,10 respectively. The ovarian cancer risk among patients with Lynch syndrome is 12%.11 And Lynch syndrome is increased endometrial cancer by 42% to 54%. However, someone with no family history of Lynch syndrome affected endometrial cancer was reported.12 The most two affected genes are mutL homolog 1 (MLH1; 40-45% of cases), mutS homolog 2 (MSH2; 40-45%).13
Some studies on the genetic background of BOT were recently reported. A small proportion of BOT patients are known to have a somatic mutation.14 Another genetic test for BOT showed the possibility of an additional treatment based on gene sequencing results.15 However, little is known about germ line mutations in BOT, therefore in this study we assessed the hereditary risk of BOT based on immunohistochemistry (IHC) analysis.
Thirty-four women with BOT and available tumor blocks were identified among patients being treated in department of Obstetrics and Gynecology, Samsung Chang-won Hospital since 2003 and 2013.
After Institutional Review Board (IRB) approval (IRB No: 2012-SCMC-028-00), family history, clinical characteristics were collected through medical charts review. IHC was performed on all tumor specimens to determine the protein expression of BRCA1, BRCA2, MSH2, and MLH1. For IHC analysis monoclonal antibodies against MSH2 (Novocastra Laboratories Ltd., Newcastle upon Tyne, UK), MLH1 (Novocastra Laboratories Ltd., Newcastle upon Tyne, UK), BRCA1 (Abcam Ltd., Cambridge, UK), and BRCA2 (Abcam Ltd., Cambridge, UK) were used. Immunostaining was performed using the Bond-Max immunostainer (Leica Biosystems Newcastle Ltd., Newcastle Upon Tyne, UK) according to the manufacturer's instructions. Normal staining patterns for MSH2, MLH1, BRCA1, and BRCA2 were nuclear staining. Loss of expression in cancer cells was demonstrated by the total absence of nuclear staining in the tumor. Adjacent normal stroma or infiltrating lymphocytes served as an internal positive control for each case.
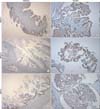
All cases were evaluated by dedicated two gynecologic pathologists two times. Staining was scored based on the intensity and proportion as follows: negative (or 0 and 1): intensity undetectable or minimal, proportion < 5%; 1+: intensity mild, proportion 5-30%; positive (or 2+): intensity moderate to marked, proportion 30-100% (Fig. 1). Two cases were excluded from analysis, one had only pathologic slides and the other lacked BRCA1 IHC analysis.
Finally, 32 cases were analyzed for the following demographic characteristics: age, menopause, parity, CA-125 level, and progression-free survival (PFS). We used chi-square or Fisher exact test for categorical variables. A P value < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS software (version 12.0; SPSS Inc., Chicago, IL, USA).
Among the 34 cases with available tumors, two cases were excluded due to incomplete data. The demographic characteristics for the 32 patients are listed in Table 1. The median age at diagnosis was 44 years (range, 19 to 86 years), and 81% of cases were older than 30 years. Eight women (25%) were premenopausal and six (19%) were nulliparous. Among 31 cases, 16 (57%) had abnormal cancer antigen 125 (CA-125; level > 35 U/mL) before surgery. Among patients with an abnormal CA-125 level the pathologic distribution was 63% serous type and 37% mucinous type. Analysis of family history of the patients showed no family cancer history in their medical charts. The median follow up period was 21.8 months (range 0-105) and no cases showed recurrence. The median PFS was 21.8 months (range 0-105).
Regarding histology, 14 patients (44%) were serous type, and 18 (56%) were mucinous type (Table 2).
Patients were classified into three groups by operation method: unilateral ovarian cystectomy (UOC), unilateral salpingo-oophorectomy (USO), and bilateral salpingo-oophorectomy (BSO) including total hysterectomy. All 32 patients underwent surgery, and the number of UOC, USO, and BSO procedures was 4 (12%), 20 (63%), and 8 (25%) respectively (Table 3). UOC cases undertook re-staging operation later.
IHC analysis of four hereditary related genes was conducted on tumor blocks. None of the samples showed loss of expression, as indicated by negative IHC staining, but weak staining was found for MSH2 (n = 1, 3%) and BRCA2 (n = 6, 19%). The pathology of the patient with weak MSH2 staining was mucinous and that of the patients with weak BRCA2 staining was serous (n = 2) or mucinous (n = 3) (Table 4).
Interestingly, one patient with a serous borderline tumor was also diagnosed with a serous cystadenocarcinoma at the same time. Before the operation, her serum CA-125 was elevated (554.7 U/mL) and the computed tomography (CT) image indicated a malignant appearance for the right ovarian mass. The patient underwent BSO, total hysterectomy, pelvic lymph node dissection, total omentectomy, and an appendectomy for surgical staging. Based on surgical findings from frozen biopsy the left ovary looked normal but the right ovarian mass was malignant. The final pathologic finding was serous borderline tumor in the left ovary and serous cystadenocarcinoma in the right ovary. We obtained IHC data corresponding to both tumors, which showed the same result of weak BRCA2 staining and strong staining for the other markers for both tumors.
There were 2,124 newly detected cases of ovarian cancer and the estimated mortality was 987 in Korea.16 Theory has been proposed regarding carcinogenesis of ovarian carcinoma designated type I and type II.17,18 Type I, or borderline, tumors are low-grade serous carcinoma, mucinous carcinoma, endometrioid carcinoma, malignant Brenner tumor, and clear cell carcinoma, whereas Type II tumors are high-grade serous carcinoma. These two types differ in their pathogenesis, molecular events, behavior, and prognosis, and it is rare for a low-grade serous carcinoma to change to a high-grade serous carcinoma.17,18 But hereditary background has not been fully studied so far.
Difficulties with ovarian carcinomas are hardness to diagnose early and cost to treat them.
Most ovarian carcinoma patients do not have specific symptoms and 16% are asymptomatic at the time of diagnosis.19 As a result, when they are diagnosed the stage tends to be more advanced. In addition, treatment costs for cancer are increasing.20 Early diagnosis of ovarian cancer is difficult, but very important. We do not know exactly what proportion of BOT represent suspicious ovarian carcinoma or a hereditary cancer risk, although it is believed to be small.
BOT are a transitional category between benign and malignant. The prognosis of BOTs is generally good,21 but they can recur or change cancer type. In one study, 28 women (17%) showed recurrence as either BOT (23 womens) or carcinoma (5 womens) after fertility-sparing surgery for BOT.22 In another study, the recurrence rate of serous BOT with non-invasive implants was 44% and the mortality was 25%.5 In some cases BOT is diagnosed concurrently with serous cystadenocarcinoma, as seen in our study.
The exact hereditary risk of cancer associated with BOT is unknown. There currently is no direct evidence that BOT is associated with hereditary ovarian tumor although several studies have evaluated the pathologic features of hereditary ovarian cancer.23 Among 11 studies of HBOC, one study reported a single mucinous borderline tumor among 13 cases of cancer associated with BRCA1 mutation.24 The pathologic features of Lynch syndrome patients indicate that more than 90% of the tumors are carcinomas, with borderline tumors representing only 4.1% of the epithelial cancers.25
Therefore, there is a possible association between HBOC or Lynch syndrome and BOT. Many studies of BOT are ongoing, but few are evaluating the hereditary risk. Our study reveals a potential hereditary risk of cancer among BOT patients and suggests that between 3% and 19% of patients may need genetic counseling and confirmative testing.
Our study is the first preliminary study of the hereditary risk of BOT using IHC. Our diagnosis was based on several clinical and IHC criteria. One previous study showed a strong correlation between BRCA1 IHC data and molecular events in ovarian cancer26 and another study showed the feasibility of IHC for detecting Lynch syndrome.27 For initial screening of Lynch syndrome the most commonly used test is IHC with 82.6% in endometrial tumor screening and 64.2% in colorectal tumor screening.28
A recent study involving whole exome analysis of low-grade serous ovarian carcinomas identified an average of only 10 somatic mutations per tumor in seven cases.29 Another study that examined the entire exome of serous BOTs for somatic genetic mutations showed similar results to low-grade serous carcinomas14. We should therefore consider hereditary risk, rather than somatic mutation.
There are studies for markers of ovarian tumor. Expression of p53 and Jab1 proteins is showed positive trend of ovarian cancer, but expression of p27 protein is related negative effect.30 In other hands, benign tumor, such as endometriosis, is associated Estrogen receptor gene polymorphisms.31
Our studies indicate the need for a large prospective stu dy of BOT. Further studies should evaluate the cost effectiveness and appropriate selection of candidates for genetic testing. We also recommend MLH1, MSH2, BRCA1, and BRCA2 IHC analysis during operative pathology for patients with ovarian masses, and cooperation and close follow-up with a pathologist.
In summary, our study is the first report of the risk of hereditary borderline ovarian cancer in gynecologic malignancy patients in Korea based on clinical and IHC criteria (MLH1, MSH2, BRCA1, and BRCA2 protein expression). We found that 3% and 19% of women with borderline ovarian cancer had relevant MSH2 and BRCA2 IHC data respectively, suggesting that a small proportion of patients might need genetic counseling and gene sequencing for hereditary risk evaluation. Identification of patients with borderline cancer through the acquisition of family history, IHC, and CA-125 data can prepare us for better consultation and might prevent the development of more advanced cancers.
Figures and Tables
Fig. 1
Immunohistochemical staining of BRCA1, BRCA2, and MSH2. (BRCA1: breast cancer 1, early onset, BRCA2: breast cancer 2, early onset, MSH2: mutS homolog 2)
Acknowledgment
This study was supported by a grant from the Samsung Biomedical Research Institute (SMR112162).
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
2. Swanton A, Bankhead CR, Kehoe S. Pregnancy rates after conservative treatment for borderline ovarian tumours: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2007; 135:3–7.
3. Gershenson DM. Clinical management potential tumours of low malignancy. Best Pract Res Clin Obstet Gynaecol. 2002; 16:513–527.
4. Lalwani N, Shanbhogue AK, Vikram R, Nagar A, Jagirdar J, Prasad SR. Current update on borderline ovarian neoplasms. AJR Am J Roentgenol. 2010; 194:330–336.
5. Silva EG, Gershenson DM, Malpica A, Deavers M. The recurrence and the overall survival rates of ovarian serous borderline neoplasms with noninvasive implants is time dependent. Am J Surg Pathol. 2006; 30:1367–1371.
6. Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Kwan E, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001; 68:700–710.
7. Narod SA, Boyd J. Current understanding of the epidemiology and clinical implications of BRCA1 and BRCA2 mutations for ovarian cancer. Curr Opin Obstet Gynecol. 2002; 14:19–26.
8. Whittemore AS, Gong G, Itnyre J. Prevalence and contribution of BRCA1 mutations in breast cancer and ovarian cancer: results from three US population-based case-control studies of ovarian cancer. Am J Hum Genet. 1997; 60:496–504.
9. Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994; 266:66–71.
10. Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995; 378:789–792.
11. Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999; 81:214–218.
12. Heo EJ, Park JM, Lee EH, Lee HW, Kim MK. A case of perimenopausal endometrial cancer in a woman with MSH2 germline mutation. J Menopausal Med. 2013; 19:143–146.
13. Burgart LJ. Testing for defective DNA mismatch repair in colorectal carcinoma: a practical guide. Arch Pathol Lab Med. 2005; 129:1385–1389.
14. Boyd J, Luo B, Peri S, Wirchansky B, Hughes L, Forsythe C, et al. Whole exome sequence analysis of serous borderline tumors of the ovary. Gynecol Oncol. 2013; 130:560–564.
15. Varga J, Bilecová-Rabajdová M, Ostró A. Borderline ovarian tumor - a case report with genetic testing. Eur J Gynaecol Oncol. 2013; 34:189–192.
16. Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012; 44:25–31.
17. Dehari R, Kurman RJ, Logani S, Shih Ie M. The development of high-grade serous carcinoma from atypical proliferative (borderline) serous tumors and low-grade micropapillary serous carcinoma: a morphologic and molecular genetic analysis. Am J Surg Pathol. 2007; 31:1007–1012.
18. McCluggage WG. The pathology of and controversial aspects of ovarian borderline tumours. Curr Opin Oncol. 2010; 22:462–472.
19. Webb PM, Purdie DM, Grover S, Jordan S, Dick ML, Green AC. Symptoms and diagnosis of borderline, early and advanced epithelial ovarian cancer. Gynecol Oncol. 2004; 92:232–239.
20. Shin JY, Kim SY, Lee KS, Lee SI, Ko Y, Choi YS, et al. Costs during the first five years following cancer diagnosis in Korea. Asian Pac J Cancer Prev. 2012; 13:3767–3772.
21. Seidman JD, Kurman RJ. Ovarian serous borderline tumors: a critical review of the literature with emphasis on prognostic indicators. Hum Pathol. 2000; 31:539–557.
22. Zanetta G, Rota S, Lissoni A, Meni A, Brancatelli G, Buda A. Ultrasound, physical examination, and CA 125 measurement for the detection of recurrence after conservative surgery for early borderline ovarian tumors. Gynecol Oncol. 2001; 81:63–66.
23. Prat J, Ribé A, Gallardo A. Hereditary ovarian cancer. Hum Pathol. 2005; 36:861–870.
24. Stratton JF, Gayther SA, Russell P, Dearden J, Gore M, Blake P, et al. Contribution of BRCA1 mutations to ovarian cancer. N Engl J Med. 1997; 336:1125–1130.
25. Watson P, Butzow R, Lynch HT, Mecklin JP, Jarvinen HJ, Vasen HF, et al. The clinical features of ovarian cancer in hereditary nonpolyposis colorectal cancer. Gynecol Oncol. 2001; 82:223–228.
26. Garg K, Levine DA, Olvera N, Dao F, Bisogna M, Secord AA, et al. BRCA1 immunohistochemistry in a molecularly characterized cohort of ovarian high-grade serous carcinomas. Am J Surg Pathol. 2013; 37:138–146.
27. Kim MK, Song SY, Do IG, Kim SH, Choi CH, Kim TJ, et al. Synchronous gynecologic malignancy and preliminary results of Lynch syndrome. J Gynecol Oncol. 2011; 22:233–238.
28. Cohen SA. Current Lynch syndrome tumor screening practices: a survey of genetic counselors. J Genet Couns. 2014; 23:38–47.
29. Jones S, Wang TL, Kurman RJ, Nakayama K, Velculescu VE, Vogelstein B, et al. Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol. 2012; 226:413–420.
30. Lee WS, Park ES, Kim DH, Kim TH, Lee HH, Chung SH. Expression of p53, p27 and Jab1 protein in epithelial ovarian tumors. Eur J Gynaecol Oncol. 2012; 33:358–362.
31. Mun MJ, Kim JH, Kim TH, Hwang JY, Jang WC. Associations between estrogen receptor gene polymorphisms and endometriosis. J Korean Soc Menopause. 2013; 19:64–73.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download