TO THE EDITOR: Myelodysplastic syndrome (MDS) is a heterogeneous group of hematopoietic stem cell disorders with ineffective hematopoiesis and resulting cytopenias [1]. Approximately 10–20% of patients with MDS present with autoimmune diseases, such as vasculitis, rheumatoid arthritis, and inflammatory bowel disease [23]. Sometimes, patients with MDS develop skin manifestations including pyoderma gangrenosum (PG), Sweet syndrome, and skin infiltration of malignant cells. The appearance of cutaneous lesions is associated with a poor prognosis [456]. Traditionally, hematopoietic stem cell transplantation (HSCT) has been used for refractory autoimmune diseases [789] and hematological disorders. Here, we report a case of MDS associated with PG and Behçet's disease that was successfully treated with allogeneic stem cell transplantation.
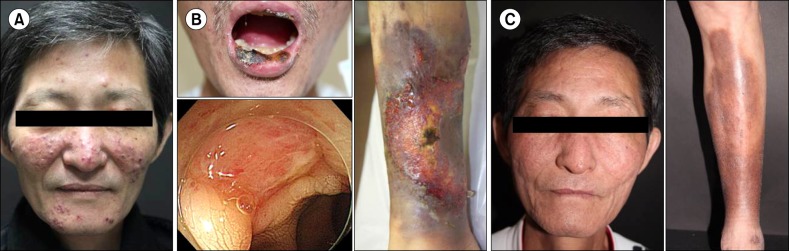
A 53-year-old man was referred to our hospital in December 2011 for evaluation of pancytopenia. He had suffered from a chronic granulomatous skin lesion on the face for 2 years; there was no improvement despite treatment with methylprednisolone, methotrexate, and dapsone (Fig. 1A). Laboratory findings were as follows: white blood cell count 1,900/µL, neutrophils 1,010/µL, hemoglobin 8.5 g/dL, platelets 112,000/µL, and no blasts in the peripheral blood. At this time, a minimal dose of methotrexate (5 mg/week) was being administered. A bone marrow biopsy showed 4.6% myeloblasts and increased megakaryocytes with dysplastic features. We could not get cytogenetic information due to an inappropriate specimen for karyotyping.
He was diagnosed with MDS with refractory cytopenia with multilineage dysplasia (RCMD) and received a packed red blood cell transfusion every 2 months. In December 2013, he was hospitalized with fever, an aggravated skin lesion, and severe cytopenias (Fig. 1B). Lab tests revealed aggravated pancytopenia: white blood cell count 2,000/µL, neutrophils 240/µL, hemoglobin 4.9 g/dL, and platelets 32,000/µL.
A colonoscopy, performed to evaluate the marked anemia, showed an ulcerative lesion in the terminal ileum (Fig. 1B). A biopsy revealed a chronic ulcer with active inflammation and polymerase chain reaction tests for Mycobacterium tuberculosis were negative. Serological tests revealed positivity for HLA B51 and a pathergy test was positive. Based on these results, he was diagnosed with Behçet's disease (Fig. 1B). A skin biopsy of the lower lip showed ulceration with acute and chronic inflammation and focal suppurative inflammation, suggestive of pyoderma gangrenosum.
A subsequent examination of his bone marrow showed a persistent state of MDS with RCMD with a karyotype of 47, XY, dup(1)(q21q32), +8[20]. Based on the International Prognostic Scoring System (IPSS), he was categorized into the intermediate-1 risk group; based on the revised IPSS, he was categorized into the high-risk group. He received three cycles of azacitidine (75 mg/m2 intravenously for 7 days, every 4 wk) bridge therapy before HSCT, which did not lead to a response. Unrelated peripheral blood HSCT with reduced-intensity conditioning (30 mg/m2/day fludarabine intravenously for 6 days; 3.2 mg/kg/day busulfan intravenously for 2 days; total 5 mg/kg anti-thymocyte immunoglobulin for graft-versus-host-disease prophylaxis) was performed in March 2014, 27 months after the diagnosis of MDS. At the time of transplantation, the PG lesion on the leg partially improved, but the facial PG lesion was still active. Neutrophil engraftment occured on day 17, and platelet engraftment on day 27. About one year after transplantation, ulcerative skin lesions on the face and lower leg disappeared (Fig. 1C) and follow-up colonoscopy revealed no abnormal findings in the terminal ileum. Since then, up to the present, he is free of transfusions and immunosuppressants.
In this case of MDS involving trisomy 8, cutaneous manifestations (i.e., PG) preceded an overt hematological disorder and Behcet's disease. PG was refractory to several immunosuppressants but responsive to MDS treatment with allogeneic HSCT. Several case reports and series have revealed associations between various skin lesions and MDS. Farah et al. [5] reported that up to 10% of patients diagnosed with MDS experienced skin manifestations, including nonspecific neutrophilic dermatosis, specific-lesion (neoplastic hematopoietic cell infiltration) and leukocytoclastic vasculitis. Haga et al. [10] demonstrated that the neutrophils in neutrophilic dermatosis originated from MDS cells. Survival analysis showed a trend of increased leukemic transformation in patients with skin lesions. Thus, it is important to recognize skin lesions in MDS patients even in the early stage, and they warrant close observation.
In patients with underlying diseases, therapy should be directed not only to autoimmune disease but also to the systemic disease. In this case, hypomethylating agent (HMA) treatment did not improve pancytopenia or the skin lesions. It is unclear whether responsiveness to HMA treatment is associated with improvements in skin manifestations. Considering the favorable clinical outcome after HSCT in this patient, an aggressive treatment strategy may be needed, if feasible, in intermediate-risk MDS patients with skin manifestations.
In conclusion, our case provides important evidence indicating that allogeneic HSCT can be a curative modality for the treatment of MDS and associated pyoderma gangrenosum and Behcet's disease.
References
1. Komrokji RS, Zhang L, Bennett JM. Myelodysplastic syndromes classification and risk stratification. Hematol Oncol Clin North Am. 2010; 24:443–457. PMID: 20359636.

2. Al Ustwani O, Ford LA, Sait SJ, et al. Myelodysplastic syndromes and autoimmune diseases-case series and review of literature. Leuk Res. 2013; 37:894–899. PMID: 23692654.
3. Komrokji RS, Kulasekararaj A, Al Ali NH, et al. Autoimmune diseases and myelodysplastic syndromes. Am J Hematol. 2016; 91:E280–E283. PMID: 26875020.

4. Hagiwara A, Fujimura T, Furudate S, et al. Generalized granulomatous dermatitis accompanied by myelodysplastic syndrome. Acta Derm Venereol. 2014; 94:223–224. PMID: 23817627.

5. Farah C, Bulai Livideanu C, Jegu J, et al. Prevalence and prognostic value of cutaneous manifestations in patients with myelodysplastic syndrome. J Eur Acad Dermatol Venereol. 2010; 24:1171–1175. PMID: 20202054.

6. Avivi I, Rosenbaum H, Levy Y, Rowe J. Myelodysplastic syndrome and associated skin lesions: a review of the literature. Leuk Res. 1999; 23:323–330. PMID: 10229317.

7. Tyndall A, Daikeler T. Autologous hematopoietic stem cell transplantation for autoimmune diseases. Acta Haematol. 2005; 114:239–247. PMID: 16269864.

8. Bohgaki T, Atsumi T, Koike T. Autoimmune disease after autologous hematopoietic stem cell transplantation. Autoimmun Rev. 2008; 7:198–203. PMID: 18190878.

9. Yamanaka K, Kuniyuki S, Maekawa N, Yoshida Y, Teshima H. Pyoderma gangrenosum with myelodysplastic syndrome treated with analogous bone marrow transplantation. Acta Derm Venereol. 2009; 89:105–106. PMID: 19197560.

10. Haga N, Iwata H, Yamaguchi Y, et al. Mucocutaneous pyoderma gangrenosum due to trisomy 8 neutrophilic infiltrates in a patient with myelodysplastic syndrome. Br J Dermatol. 2016; 174:239–241. PMID: 26301955.

Fig. 1
Presentation of clinical course after diagnosis of myelodysplastic syndrome (MDS). The facial granulomatous lesion at diagnosis (A), oral ulcer, suppurative ulcer on leg, and ulcerative lesion in ileum by colonoscopy (B). Improvement of the facial granulomatous lesion and leg ulcer after allogeneic stem cell transplantation (SCT) (C).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download