Abstract
Background
We investigated the molecular epidemiological characteristics and antimicrobial susceptibility pattern of penicillinase-producing Neisseria gonorrhoeae (PPNG) isolates to monitor the change in distribution of blaTEM in Korea.
Methods
We collected 804 PPNG isolates from diverse hospitals and clinics mainly located in Seoul, Korea, over a period of 11 years (2005–2015). Isolate susceptibility to seven antimicrobials was determined using the agar dilution test. The molecular epidemiological characteristics of the isolates were determined by Sanger sequencing of blaTEM, N. gonorrhoeae multiantigen sequence typing (NG-MAST) and plasmid typing.
Results
Among 72 fully sequenced PPNG isolates, sixteen (22.2%) possessed TEM-135. All TEM-135 isolates had a common silent mutation (c.18C>T), which was previously unreported. We observed a pattern of continuous increase in the number of TEM-135 isolates since 2012. The median and 90% minimum inhibitory concentration of azithromycin were substantially lower in the TEM-135 group than in the non-PPNG and TEM-1 groups. All TEM-135 isolates showed different NG-MAST types and predominantly harbored Toronto/Rio (75%) plasmids. A comprehensive comparative analysis of PPNG with TEM-135 according to NG-MAST, plasmid type, and year of isolation revealed a wide distribution.
Conclusions
The proportion of TEM-135 PPNG has continuously increased since 2012, in association with clonal spread. The difference at position 18 of the TEM-135 sequence can be interpreted as the existence of multiple clonal complexes. The possibility that TEM-135 was acquired via foreign plasmids requires careful follow-up and continuous monitoring of TEM-135 to ascertain whether it constitutes a step towards evolutionary change.
Penicillin treatment failure was first reported soon after the introduction of penicillin administration in the 1940s; dissemination of penicillin resistance has made this antibiotic useless since the 1950s [1]. The mechanism of penicillin resistance prevalent during the 1960s and mid-1970s is associated with mutations in chromosomal genes (mainly penA gene) encoding for penicillin binding proteins (chromosomally-mediated penicillin resistant N. gonorrhoeae, CMRNG) [12]. Although CMRNG resulted in clinical treatment failure, administration of high-dose penicillin remained effective against CMRNG until a new resistance mechanism emerged in 1976 [3]. This novel mechanism resulted from the acquisition of a plasmid-located β-lactamase gene (blaTEM-1) exhibiting high-level resistance to penicillin (penicillinase-producing N. gonorrhoeae, PPNG). Wide dissemination of PPNG ultimately provoked the usage of extended-spectrum cephalosporin antibiotics, including ceftriaxone and cefixime, which were effective against the TEM-1 β-lactamase with a narrow hydrolytic spectrum [45]. However, there have been frequent recent reports of new β-lactamase variants sharing common single nucleotide polymorphisms (compared with TEM-1).
TEM-135, which has a single amino acid change (M182T) compared with the TEM-1 sequence, was first reported in 2005 [6], and its frequency and genetic diversity have been consistently evaluated in many countries. The clinical significance of TEM-135 remains unclear, as there are no major differences in the minimal inhibitory concentrations (MICs) of penicillin and extended-spectrum cephalosporins; however, the emergence of TEM-135 could signify the introduction of other TEM β-lactamases with an extended β-lactam hydrolytic spectrum through mutation or plasmid acquisition under the high selective pressure of cephalosporin usage. We aimed to determine the prevalence of TEM-135 and compare the characteristics of TEM-1- and TEM-135-producing N. gonorrhoeae isolated in Korea from 2005 to 2015.
A total of 804 isolates were collected over 11 years (2005–2015) from 40 diverse hospitals and primary-care clinics in Korea to test for the presence of the TEM-135 gene. This study was approved by the Institutional Review Board of the Human Research Protection Center in the Yonsei University Health System (4-2016-0359). Transgrow medium was used as the transport medium to minimize the loss of viable N. gonorrhea isolates, and modified Thayer-Martin medium (Becton-Dickinson, Cockeysville, MD, USA) was used for colony growth and incubation. Bacterial identification was confirmed by Gram-stain and the Vitek system (bioMerieux, Marcy l'Etoile, France) or by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Bremen, Germany). All isolates were collected for the national surveillance program for gonococci supported by the Korean Centers for Disease Control and Prevention. They were stored at −70℃ until the number of isolates collected was sufficient for analysis. Molecular analysis was performed.
The antimicrobial susceptibility of all isolates to the antibiotics (penicillin G, tetracycline, ceftriaxone, spectinomycin, ciprofloxacin, nalidixic acid, and azithromycin) was determined using the CLSI disk diffusion method [7]. MIC was calculated using the agar dilution test according to the CLSI guidelines [8] for all antibiotics except azithromycin, for which the European Committee on Antimicrobial Susceptibility Testing (EUCAST) cut-off values were used [9]. The median and 90% MIC values (MIC50 and MIC90) and range were evaluated for the three groups of isolates: non-PPNG, PPNG with TEM-1, and PPNG with TEM-135.
DNA was extracted from PPNG bacterial suspensions, using the QIAquick gel extraction kit (Qiagen, Hilden, Germany). Mismatch amplification mutation assay (MAMA) PCR was performed to detect the blaTEM-135 allele, and TEM PCR was performed to identify both blaTEM-1 and blaTEM-135 [10]. The complete blaTEM sequences of PPNG isolates were confirmed by Sanger sequencing based on a comparison between the TEM-135 reference sequence (GenBank accession number: GQ896333) and the PCR products.
To evaluate the molecular epidemiology of the TEM-135 isolates, NG-MAST was performed by sequencing the porB and tbpB genes; allele numbers and sequence types (STs) were assigned using the NG-MAST website (http://www.ng-mast.net), as previously described [11]. For plasmid typing, multiplex PCR was performed on all isolates, as previously described, using primers BL1, BL2, BL3, and BL4 [12].
PPNG isolates accounted for 11.6% (N=93) of the 804 isolates, with patterns fluctuating by collection year (Fig. 1). Only 72 PPNG isolates (77.4%) were available for further molecular investigation because of loss or deterioration of the isolates during the storage period. These 72 isolates were subjected to full sequence analysis to determine the presence of TEM-1 or TEM-135; 22.2% (N=16) were shown to harbor TEM-135. The first TEM-135 isolate was identified in 2006, and the number of TEM-135 isolates has continuously increased since 2012. Although no variant types other than TEM-1 and TEM-135 were found, all isolates possessed a silent mutation, c.18C>T, which does not change the original amino acid (Proline) compared with the previously reported blaTEM-135 reference sequence (GQ896333; Table 1).
Of the 72 PPNG isolates, 52 (38 TEM-1 and 14 TEM-135 isolates) were randomly selected (mainly because of strain availability) for antimicrobial susceptibility testing with seven antibiotics. The proportions of susceptible isolates for the seven antibiotics were similar between the TEM-1 group and TEM-135 group, whereas the non-PPNG group showed a different pattern (Table 2). Comparison of the MIC50 and MIC90 (determined using the agar dilution test) of the three groups (non-PPNG, PPNG with TEM-1, and PPNG with TEM-135) showed that the MIC50 and MIC90 of ceftriaxone and cefixime were significantly lower in the TEM-135 than in the other groups (all values ≤0.008 µg/mL). Additionally, the MIC50 and MIC90 of azithromycin in the TEM-135 group were also lower than those in the TEM-1 group (MIC50=0.06 µg/mL and MIC90=0.25 µg/mL for the TEM-135 group). No significant changes in antimicrobial resistance trends were observed in the TEM-1, TEM-135, and non-PPNG groups (Supplemental Data Table S1).
The STs of 72 PPNG isolates were determined by NG-MAST in order to elucidate the molecular epidemiology of plasmids possessing certain types of penicillinases; 59 different STs were identified, indicating heterogeneous epidemiological distribution (Fig. 2). The STs bearing TEM-135 were dispersed throughout the genetic tree (generated based on por gene sequences using the unweighted pair group method with arithmetic mean [UPGMA] approach), except for two small clusters (cluster A and B; Fig. 2). Plasmid typing of all PPNG isolates demonstrated that the African plasmid type was the most common type, present in 51/72 isolates (70.8%), followed by the Toronto/Rio (N=13, 18.1%) and the Asian (N=8, 11.1%) plasmid types. However, among the TEM-135 isolates, the Toronto/Rio plasmid type was predominant (N=13, 81.3%), followed by the Asian (N=2, 12.5%) and the African (N=1, 6.2%) plasmid types. ST3965 isolates all possessed TEM-1 on an African type plasmid, except for one isolate with TEM-135 on a Toronto/Rio type plasmid. In cluster A, five TEM-135 isolates (designated with black dots and bold red numbers; blue box, Fig. 2) appeared to be closely related based on close genetic distances (determined by NG-MAST), the predominance of the Toronto/Rio type (established by plasmid typing), and recent isolation (within the last three years). Four isolates in cluster B (Fig. 2) harbored three Toronto/Rio plasmids and one Asian plasmid. Interestingly, the Asian and African plasmids bearing TEM-135 have appeared recently (in 2012, 2014, and 2015).
The emergence of PPNG was one reason for the use of extended-spectrum cephalosporins, including ceftriaxone and cefixime; the appearance of TEM-135 could constitute another turning point in gonococcal treatment through the evolution or introduction of novel β-lactamases to N. gonorrhoeae. Following the first report of TEM-135 in Japan, the reported prevalence of TEM-135 ranged from 9.4% to 57.8% according to country [101314151617]. The average prevalence of TEM-135 in our study was 22.2% over a period of 11 years (2005–2015). Although the prevalence trends showed fluctuations, a pattern of continuous increase in the proportion of TEM-135 is observed in the last four years (2012–2015).
The antimicrobial susceptibility patterns of ceftriaxone, cefixime, azithromycin, and tetracycline in PPNG with TEM-135 differed from those in PPNG with TEM-1. Although all PPNG isolates were susceptible to ceftriaxone and cefixime, the MIC50 and MIC90 of both antibiotics were much lower in PPNG with TEM-135 (both values ≤0.008 µg/mL) than in PPNG with TEM-1 (0.03 µg/mL and 0.12 µg/mL, respectively). The rate of azithromycin-susceptible isolates was 100% in TEM-135 strains, but 60% in TEM-1 strains. The MIC50 of tetracycline in TEM-135 and TEM-1 PPNG was 0.5 µg/mL and 4 µg/mL, respectively, and the rates of resistance were 30.8% and 78.1%, respectively. Although a previous study conducted in England utilizing a similar approach discovered statistically higher ciprofloxacin and tetracycline MIC values in TEM-135 strains [14], our results do not support such trends. However, they may suggest clonal relatedness consisting of isolates highly susceptible to ceftriaxone and cefixime that can be discriminated from TEM-1 PPNG, despite the high selective pressure of ceftriaxone usage in Korea.
This hypothesis can be partially supported by our phylogenetic and plasmid analyses. The phylogenetic tree based on partial porB sequences revealed that some of the TEM-135 isolates appear to be closely related in terms of genetic distances and recent isolation within the last three years (Cluster A and B; Fig. 2). However, the other genetically dispersed TEM-135 PPNG isolates were also highly susceptible to ceftriaxone and cefixime, which may suggest that TEM-135 PPNG isolates originated in a low ceftriaxone selective pressure environment (similar to tetracycline-resistant N. gonorrhoeae [TRNG] that has increased recently in Korea [18]), compared with TEM-1 PPNG, which is less susceptible to ceftriaxone and cefixime.
The predominance of the Toronto/Rio plasmid type in TEM-135 PPNG revealed in our study is in line with previous epidemiological studies conducted in other countries [10151619], although only one study from England reported the predominance of the Asian plasmid type [14]. Therefore, we assume that a specific plasmid (Toronto/Rio) has been distributed worldwide. The ceftriaxone and cefixime MIC values of isolates with an Asian plasmid type (0.06 µg/mL, respectively) were higher than the values of isolates with other plasmid types; however, the number of isolates was too small to draw any definitive conclusions.
A recent report identified four novel TEM alleles from globally collected strains [19]; we did not identify any new TEM alleles other than TEM-1 and TEM-135. Interestingly, all isolates in this study had a thymidine instead of cytosine at nucleic acid position 18 compared with the previously reported blaTEM-135 reference sequence from Thailand (GenBank accession number: GQ896333). However, a comparison of our sequences with N. gonorrhoeae TEM-1 and TEM-220 sequences revealed that thymidine at position 18 is common (Table 1). Moreover, the blaTEM-135 sequence in Salmonella enterica subsp. enterica serovar Typhimurium, in which TEM-135 was first identified, was identical to that of all our TEM-135-harboring isolates. This sequence is also identical to the sequences of the first TEM-135 isolates reported in Japan [17]. Thus, the TEM-135 PPNG isolates from our study might be more closely related to the original Japanese TEM-135 isolates than to the previously reported reference strain. Furthermore, the clonal relatedness of TEM-135 strains only became prominent in the last three years, suggesting the possibility of foreign acquisition.
In conclusion, the proportion of TEM-135 PPNG has continuously increased during the past four years and most of the isolates are highly susceptible to ceftriaxone and cefixime despite the high selective pressure of ceftriaxone usage in Korea. The recent increase in TEM-135 PPNG proportion is associated with clonal spread; however, we hypothesize that most of the isolates have originated from relatively low ceftriaxone selective pressure environments. Moreover, the difference at position 18 of the TEM-135 sequence can be interpreted as the presence of at least two clonal complexes, one of which is thought to be associated with the Japanese sequence, even though direct connection cannot be proved. The possibility of foreign import requires rigorous follow-up to control the foreign acquisition of novel resistance and continuous monitoring of TEM-135 to elucidate whether TEM-135 constitutes a step towards evolutionary change of resistance to prevent the dissemination.
Figures and Tables
Fig. 1
Chronological changes in the percentage of isolates with TEM-1 or TEM-135 β-lactamase among all the N. gonorrhoeae isolates collected between 2005 and 2015.

Fig. 2
Genetic distance tree and plasmid types of penicillinase-producing Neisseria gonorrhoeae with TEM-1 or TEM-135 grouped according to porB genotype. Alleles marked with a black circle or numbers shown in red are associated with isolates harboring TEM-135; numbers in parenthesis indicate the number of isolates with the same sequence type. Although sequence types are diverse among isolates with TEM-135, two small clusters (cluster A and B) accounting for 56.3% (9/16) of all TEM-135 isolates appear to be independently segregated.
Abbreviation: NG-MAST, Neisseria gonorrhoeae multiantigen sequence typing; ST, sequence type; AF, African; AS, Asian; TR, Tronto/Rio.

Table 2
Comparison of resistance proportion and antibiotic geometric mean MIC values according to the presence of TEM-1 or TEM-135
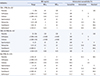
*Of the 56 TEM-1 PPNG isolates, 38 isolates were available for antimicrobial susceptibility testing with all seven antibiotics; †EUCAST cut-off values were utilized because there are no recommended cut-off values in CLSI M100-S25; ‡Of the 16 TEM-135 PPNG isolates, 14 isolates were available for antimicrobial susceptibility testing with all seven antibiotics.
Abbreviations: MIC, minimum inhibitory concentration; MIC50, median MIC; MIC90, 90% MIC; PPNG, penicillinase-producing N. gonorrhoeae.
References
1. Unemo M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014; 27:587–613.
2. Ng LK, Martin I, Lau A. Trends of chromosomally mediated antimicrobial resistance in Neisseria gonorrhoeae in Canada: 1994–1999. Sex Transm Dis. 2003; 30:896–900.
3. Ashford WA, Golash RG, Hemming VG. Penicillinase-producing Neisseria gonorrhoeae. Lancet. 1976; 2:657–658.
4. Unemo M, Del Rio C, Shafer WM. Antimicrobial resistance expressed by Neisseria gonorrhoeae: a major global public health problem in the 21st century. Microbiol Spectr. 2016; 4:EI10-0009-2015.

5. Bergstrom S, Norlander L, Norqvist A, Normark S. Contribution of a TEM-1-like beta-lactamase to penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1978; 13:618–623.
6. Pasquali F, Kehrenberg C, Manfreda G, Schwarz S. Physical linkage of Tn3 and part of Tn1721 in a tetracycline and ampicillin resistance plasmid from Salmonella typhimurium. J Antimicrob Chemother. 2005; 55:562–565.
7. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests. CLSI supplement M02-A12. 12th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2015.
8. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100-S25. 25th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2015.
9. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 5.0. 2015.
10. Nakayama S, Tribuddharat C, Prombhul S, Shimuta K, Srifuengfung S, Unemo M, et al. Molecular analyses of TEM genes and their corresponding penicillinase-producing Neisseria gonorrhoeae isolates in Bangkok, Thailand. Antimicrob Agents Chemother. 2012; 56:916–920.
11. Unemo M, Dillon JA. Review and international recommendation of methods for typing Neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin Microbiol Rev. 2011; 24:447–458.
12. Palmer HM, Leeming JP, Turner A. A multiplex polymerase chain reaction to differentiate beta-lactamase plasmids of Neisseria gonorrhoeae. J Antimicrob Chemother. 2000; 45:777–782.
13. Chen SC, Yin YP, Dai XQ, Yu RX, Han Y, Sun HH, et al. Prevalence and molecular epidemiological typing of penicillinase-producing Neisseria gonorrhoeae and their bla(TEM-135) gene variants in Nanjing, China. Sex Transm Dis. 2013; 40:872–876.
14. Cole MJ, Unemo M, Grigorjev V, Quaye N, Woodford N. Genetic diversity of blaTEM alleles, antimicrobial susceptibility and molecular epidemiological characteristics of penicillinase-producing Neisseria gonorrhoeae from England and Wales. J Antimicrob Chemother. 2015; 70:3238–3243.
15. Gianecini R, Oviedo C, Littvik A, Mendez E, Piccoli L, Montibello S, et al. Identification of TEM-135 β-lactamase in Neisseria gonorrhoeae strains carrying African and Toronto plasmids in Argentina. Antimicrob Agents Chemother. 2015; 59:717–720.
16. Whiley D, Trembizki E, Buckley C, Freeman K, Lawrence A, Limnios A, et al. Penicillinase-producing plasmid types in Neisseria gonorrhoeae clinical isolates from Australia. Antimicrob Agents Chemother. 2014; 58:7576–7578.
17. Ohnishi M, Ono E, Shimuta K, Watanabe H, Okamura N. Identification of TEM-135 β-lactamase in penicillinase-producing Neisseria gonorrhoeae strains in Japan. Antimicrob Agents Chemother. 2010; 54:3021–3023.
18. Lee H, Kim H, Kim HJ, Suh YH, Yong D, Jeong SH, et al. Increasing incidence of high-level tetracycline-resistant Neisseria gonorrhoeae due to clonal spread and foreign import. Yonsei Med J. 2016; 57:350–357.
19. Muhammad I, Golparian D, Dillon JA, Johansson A, Ohnishi M, Sethi S, et al. Characterisation of blaTEM genes and types of β-lactamase plasmids in Neisseria gonorrhoeae - the prevalent and conserved blaTEM-135 has not recently evolved and existed in the Toronto plasmid from the origin. BMC Infect Dis. 2014; 14:454.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download