Abstract
We aimed to analyze the drug resistance patterns of multidrug-resistant and extensively drug-resistant tuberculosis (TB) and the difference of drug resistance among various settings for health care in Korea. The data of drug susceptibility testing in 2009 was analyzed in order to secure sufficient number of patients from various settings in Korea. Patients were categorized by types of institutions into four groups, which comprised new and previously treated patients from public health care centers (PHC), the private sector, and Double-barred Cross clinics (DBC). The resistance rates to first-line drugs were uniformly high in every group. While the resistance rates to second-line drugs were not as high as first-line drugs, there was a pattern that drug resistance rates were lowest for PHC and highest for DBC. The differences of the resistance rates were more prominent for oral second-line drugs. Our findings implied that drug resistance to oral second-line drugs was significantly amplified during multidrug-resistant-TB treatment in Korea. Therefore, an individualized approach is recommended for treating drug-resistant-TB based on susceptibility testing results to prevent acquisition or amplification of drug resistance.
The emergence of drug-resistant tuberculosis (DR-TB) is a major obstacle for eradicating TB [1]. Multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) are well-known drug-resistant forms of TB that are difficult to treat [12]. Therefore, it is important to understand the drug resistance profiles of MDR- and XDR-TB for selecting an appropriate treatment regimen. In Korea, the rate of MDR-TB is increasing and a significant proportion of MDR-TB patients are XDR-TB [345]. However, the resistance patterns of second-line drugs in Korea have been rarely reported, and results are not consistent. Therefore, the aim of this study was to analyze the drug resistance patterns of MDR-TB and XDR-TB and the differences of drug resistance between patient groups in Korea.
In 2009, a total of 18,166 Mycobacterium tuberculosis isolates were referred to the Korean Institute of Tuberculosis (KIT) for drug susceptibility testing (DST), which is a supranational TB reference laboratory. After exclusion of duplicates, DST results of 16,860 TB patients were analyzed. Among those patients, 5,137 (4,475 new patients and 662 previously treated patients) were from public health centers (PHC). The private sector accounted for the largest proportion of patients (11,248, 66.7%). The treatment history of patients in the private sector could not be assessed because the isolates were referred to the KIT without a medical history. In addition, 475 patients were from Double-barred Cross (DBC) clinics, which belong to the Korean National Tuberculosis Association and are dedicated to treating DR-TB or chronic TB patients. Because of the institutional characteristics of DBC clinics, we assumed that patients of DBC clinics would not be newly diagnosed TB patients and have a previous treatment history.
DST was performed by using the Lowenstein-Jensen absolute concentration method at the KIT [6]. The tested drugs and their critical concentrations were as follows: 0.2 and 1.0 µg/mL for isoniazid (INH); 40 µg/mL for rifampicin (RIF), kanamycin (KM), amikacin (AMK), capreomycin (CAP) and ethionamide (ETH); 10 µg/mL for streptomycin (SM); 2 µg/mL for ethambutol (EMB) and ofloxacin (OFL); 1 µg/mL for para-aminosalicylic acid (PAS); 30 µg/mL for cycloserine (CS); and 20 µg/mL for rifabutin (RBT). Resistance to pyrazinamide (PZA) was determined by using the pyrazinamidase assay (Wayne method) as described previously [7].
Multidrug resistance (resistance to both RIF and INH) was identified in 1,341 patients (8.0%). The prevalence of MDR-TB in new patients and previously treated patients of PHCs was 3.4% and 9.5%, respectively and that at the private sector and DBC clinics was 8.0% and 48.2%, respectively (Fig. 1)
The overall drug resistance rate among the MDR-TB patients was highest in those from DBC clinics (Table 1). The resistance rates to first-line oral agents (high-level INH, RBT, EMB, and PZA) were uniformly high among the MDR-TB patients. RBT can be applicable to 28.7% of MDR-TB patients. However, the PZA resistance rates varied significantly across patient groups, ranging from 39.7% to 70.7% (P<0.001). The resistance rates to SM ranged from 20.6% to 34.4%, which were higher than those observed to other injectables. This is because SM was widely used as a first-line drug, whereas other injectables are mainly used for specifically treating MDR-TB patients. The resistance rates to injectables, such as KM, AMK, and CAP, were lowest among the tested drugs.
The MDR-TB patients showed relatively high resistance rates to OFL and Group 4 drugs (ETH, CS and PAS). In addition, differences in the prevalence of resistance to those drugs were statistically significant between patient groups (P<0.001) (Table 1). The resistance rates to OFL of private hospitals and DBC clinics were 2.3- and 3.9-times higher than those of new patients from the PHCs (Table 1). Similar drug resistance patterns were observed to Group 4 drugs and OFL. The MDR-TB patients showed an 8.3-times higher rate of CS resistance compared with that of the new patients from PHCs.
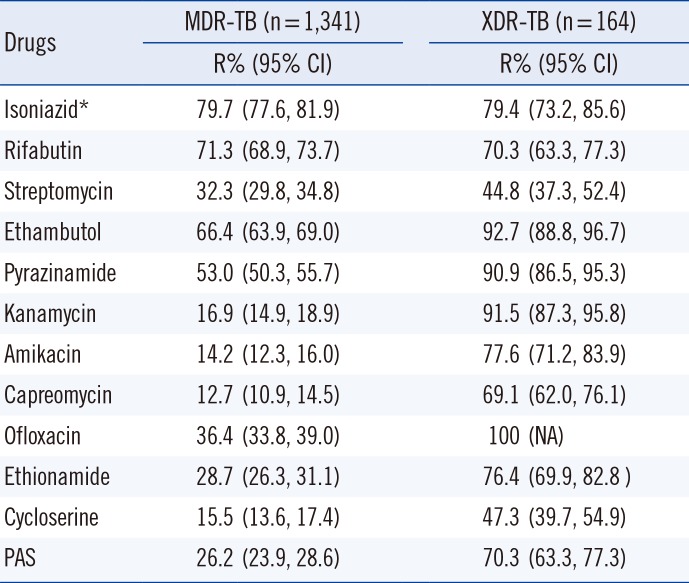
The proportion of XDR-TB among MDR-TB patients in Korea was highly variable in previous reports. Kim et al [2] reported that the proportion of XDR-TB among MDR-TB was 5.3%. However, in an analysis of the medical records of TB patients notified in 2008, Park et al [4] found that 18.8% of the MDR-TB patients were XDR-TB. Our analysis revealed that the proportion of XDR-TB varies across patient groups. XDR-TB accounted for 12.3% (165) of the MDR-TB patients ranging from 4.5% in new patients of PHCs to 20.1% in DBC clinics (Fig. 1). MDR-TB patients diagnosed at PHCs were more likely to be new MDR-TB patients, and the patients were unlikely to have been previously exposed to a regimen for treating MDR-TB. By contrast, MDR-TB patients with a previous MDR-TB treatment history or chronic patients tend to visit specialized institutes, such as DBC clinics. The majority of the XDR-TB patients were resistant to first-line oral drugs (Table 2). However, the resistance rates to high-level INH and RBT were relatively low, indicating that these drugs should be considered first for XDR-TB treatment. Among the second-line injectables, the rate of resistance to KM was highest, and the rate of resistance to CAP was lowest in every patient group. Therefore, OFL, EMB, PZA, and KM should not be included in empirical treatment regimens if XDR-TB is suspected. However, SM and CS could be the first option for treating XDR-TB because of the relatively low resistance rates to these drugs (44.8% and 47.3%). Five of the XDR-TB patients demonstrated resistance to all tested drugs, which implies that these were pandrug-resistant TB.
Our study has a limitation that we didn't include recent data for the analysis. We aimed to reveal the differences of drug resistance patterns according to types of institutions. However, TB patients notified in the private sector outnumber those in the public sector in Korea [89]. This trend has been accelerated because the Korean National Insurance System increased financial support for TB patients. Actually, more than 90% of TB patients were notified from the private sector in 2016 [9]. As a result, more than a half of DBC clinics have been closed since 2010 and the number of patients greatly declined recently. Therefore, we decided to use DST data from a year of 2009 to analyze sufficient number of patients for each group.
The private sector plays a major role in the programmatic management of DR-TB (PMDT) in Korea [8]. The majority of DR-TB patients are treated in the private sector because capacity of the public sector is limited to treat DR-TB. However, TB care in the private sector is often of poor quality owing to a lack of standardization and regulation [81011]. Thus, it is well documented that the risk of developing resistance to anti-TB drugs is higher in the private sector [11]. Therefore, it is important to analyze drug resistance patterns in both the public and private sector to best combat DR-TB. The previous reports on drugs resistance in the MDR-TB patients were unable to reflect the overall drug resistance status in Korea because such analyses were often conducted in only one sector or in a small population [24]. In the present study, the drug resistance patterns were separately analyzed for a large number of the MDR-TB patients from various patient groups.
The resistance rates to the first-line drugs were high in the MDR-TB patients from all patient groups, suggesting that the prescription of these drugs should be considered carefully according to the DST results. In contrast, the rates of resistance to second-line drugs varied substantially among the patient groups. The drug resistance rates to second-line drugs were lowest in the PHC patients and highest in those from DBC clinics. In addition, huge differences in drug resistance between patient groups were observed. This trend was more prominent for second-line oral agents, implying that the acquisition of resistance to second-line oral agents could be common during the treatment of MDR-TB. The patients of DBC clinics were resistant to more drugs than those of PHCs (8.0 vs 5.6 on average). Our results revealed that drug resistance of MDR-TB patients appears to have been amplified especially for second-line oral drugs because the likelihood of previous MDR-TB treatment was associated with higher drug resistance rates and increased number of resistant drugs. Fluoroquinolones (FQs) are the key drugs used for treating MDR-TB, and FQ resistance is closely related to a poor treatment outcome for MDR-TB [12]. Therefore, increasing FQ resistance is a serious threat to PMDT.
There are no specific treatment guidelines for XDR-TB, but the number of effective drugs is critical for treatment success [1113]. The treatment regimen for XDR-TB could be designed according to a drug resistance survey or surveillance data. However, our results showed that XDR-TB patients from Korea had high rates of resistance to most anti-TB drugs. Therefore, an individualized approach is highly recommended to treat XDR-TB based on the DST results and the patient's drug prescription history. Additional DST for Group 5 drugs may be needed to find available drugs for treating XDR-TB.
The poor management of DR-TB in Korea could results in the development of a more serious form of DR-TB. In fact, five of the XDR-TB patients were found to be resistant to all tested drugs. Fortunately, there are new options for treating DR-TB. Two new anti-TB drugs, delamanid and bedaquiline, are available, and repurposed anti-TB drugs showed good activity against TB [11]. However, we will likely face resistance to these new drugs and repeat the past without their proper and careful administration. The present study highlights the importance of aligning DST and treatment for DR-TB, and the need for strengthening PMDT.
Acknowledgments
This work was supported by the BioNano Health-Guard Research Center, funded by the Ministry of Science, ICT & Future Planning (MSIP) of Korea as a Global Frontier Project (Grant Number H-GUARD_ERND2-5).
References
1. WHO. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. http://apps.who.int/iris/bitstream/10665/44286/1/9789241599191_eng.pdf.
2. Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, et al. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med. 2008; 178:1075–1082. PMID: 18703792.
3. Bai GH, Park YK, Choi YW, Bai JI, Kim HJ, Chang CL, et al. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. Int J Tuberc Lung Dis. 2007; 11:571–576. PMID: 17439684.
4. Park YS, Hong SJ, Boo YK, Hwang ES, Kim HJ, Cho SH, et al. The national status of tuberculosis using nationwide medical records survey of patients with tuberculosis in Korea. Tuberc Respir Dis (Seoul). 2012; 73:48–55. PMID: 23101024.
5. Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martín-Casabona N, et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007; 13:380–387. PMID: 17552090.
6. Kim CK, Joo YT, Lee EP, Park YK, Kim HJ, Kim SJ. Simple, direct drug susceptibility testing technique for diagnosis of drug-resistant tuberculosis in resource-poor settings. Int J Tuberc Lung Dis. 2013; 17:1212–1216. PMID: 23823178.
7. Wayne LG. Simple pyrazinamidase and urease tests for routine identification of mycobacteria. Am Rev Respir Dis. 1974; 109:147–151. PMID: 4203284.
8. Kim JH, Yim JJ. Achievements in and challenges of tuberculosis control in South Korea. Emerg Infect Dis. 2015; 21:1913–1920. PMID: 26485188.
9. Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis in Korea, 2015. Cheongju, Korea: Korea Centers for Disease Control and Prevention;2016.
10. Zafar Ullah AN, Huque R, Husain A, Akter S, Islam A, Newell JN. Effectiveness of involving the private medical sector in the National TB Control Programme in Bangladesh: evidence from mixed methods. BMJ Open. 2012; 2(6):pii:3001534.
11. WHO. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, Switzerland: WHO;2014.
12. Yew WW, Lange C. Fluoroquinolone resistance in Mycobacterium tuberculosis. What have we learnt? Int J Tuberc Lung Dis. 2014; 18:1–2. PMID: 24365543.
13. Yuen CM, Kurbatova EV, Tupasi T, Caoili JC, Van Der Walt M, Kvasnovsky C, et al. Association between regimen composition and treatment response in patients with multidrug-resistant tuberculosis: A prospective cohort study. PLoS Med. 2015; 12:e1001932. PMID: 26714320.
Fig. 1
The prevalence of multidrug-resistant (MDR) tuberculosis (TB) and proportion of extensively drug-resistant (XDR)-TB among MDR-TB.
Abbreviations: PHC new, public health center patients with newly diagnosed tuberculosis; PHC retreatment, public health center patients with previously treated tuberculosis; DBC, Double-barred Cross.
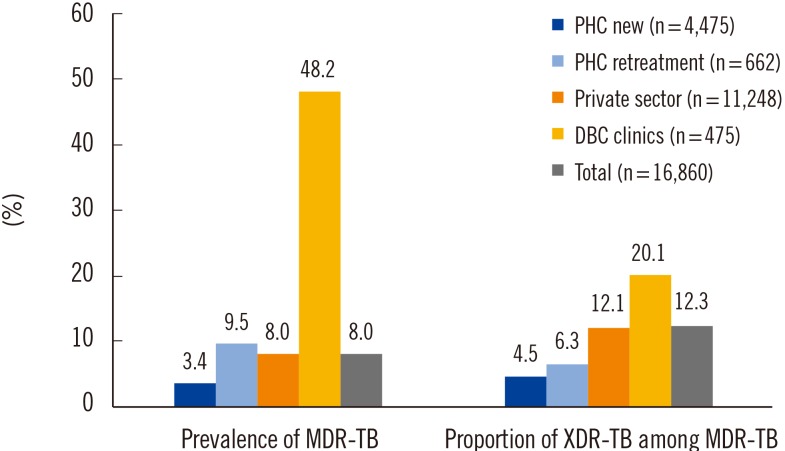
Table 1
Drug resistance in multidrug-resistant tuberculosis patients by patient group (n=1,341)
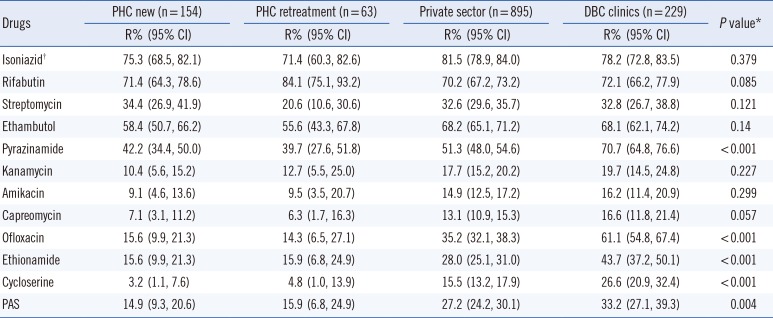
*Pearson's chi-squared test was performed to evaluate the difference in resistance rates between patient groups. A P<0.05 was considered statistically significant; †Higher concentration of isoniazid (1.0 µg/mL).
Abbreviations: R, resistance; CI, confidence interval; PHC new, public health center patients with new tuberculosis; PHC retreatment, public health center patients with previously treated tuberculosis; DBC, double-barred cross; PAS, para-aminosalicylate sodium.
Table 2
Drug resistance in multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download