INTRODUCTION
The classic Philadelphia chromosome-negative (Ph-) myeloproliferative neoplasms (MPNs) include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). In 2005, Janus kinase 2 (
JAK2) V617F mutations were detected in 95%, 50%, and 60% of PV, ET, and PMF patients, respectively [
1]. A few patients carry other
JAK2 mutations, such as insertions or deletions in exon 12 or mutations in the thrombopoietin receptor (
MPL). Approximately 40% of ET and PMF patients lack a reliable genetic marker of disease [
2]. At the end of 2013, recurrent mutations in the calreticulin (
CALR) gene were identified in two studies using whole-exome sequencing [
34]. These studies identified recurrent mutations in
CALR in 60-88% of patients with ET and PMF who were negative for
JAK2 and
MPL mutations.
CALR mutations were not found in healthy control subjects or in cases of lymphoid neoplasia, acute leukemia, or solid tumors, indicating specificity for ET and PMF [
34]. All
CALR mutations are insertions or deletions in exon 9, and the most common mutations, accounting for 80-90% of mutation cases, were either type 1, a 52-bp deletion (c.1092_1143del; p.L367fs*46), or type 2, a 5-bp insertion (c.1154_1155insTTGTC, pK385fs*47). Other infrequent mutations in exon 9 account for up to 15% of
CALR mutations [
2]. All known recurrent
CALR mutations lead to a frameshift that generates a common 36 amino acid C-terminal end, and to the loss of the KDEL motif. The distribution of
CALR mutation types differs according to the MPN type [
5].
In most previous reports,
CALR mutations were analyzed by Sanger sequencing [
3467]. However, this sequencing method is time-consuming and cannot be performed in every laboratory. Here, we present a convenient system for screening major
CALR mutations that does not require sequencing analysis. We investigated the efficiency of the screening PCR to detect
CALR mutations in the Korean patients with thrombocytosis and compared the results with those from Sanger sequencing, the reference method, and fragment analysis, a sensitive detection method.
METHODS
1. Patient selection
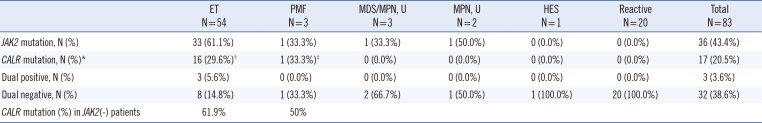
Eighty-one patients with thrombocytosis who underwent a bone marrow (BM) study at Gachon University Gil Medical Center in Korea from April 2007 to February 2015 were enrolled in this retrospective study. BM samples were obtained from patients at diagnosis (81 samples) or in a follow-up visit (2 samples). The patients included 54 with ET; three with PMF; three with myelodysplastic neoplasms (MDNs) or MPNs, unclassifiable (MDS/MPN, U); two with MPNs, unclassifiable (MPN, U); one with hypereosinophilic syndrome (HES); and 20 that were benign disease cases. The clinical and laboratory data were obtained from medical records. This study was approved by our institutional review board (GCIRB2015-73), and informed consent was obtained from all enrolled patients at that time of BM study.
2. Patient characteristics
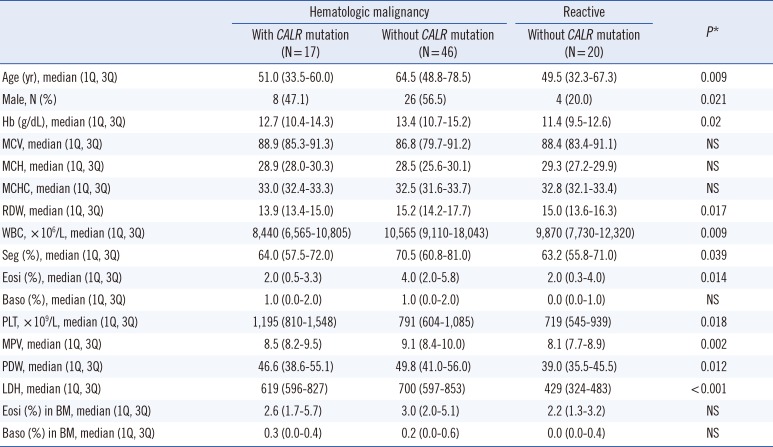
We compared the laboratory data of patients according to disease and
CALR mutation status. Statistical differences in age, sex, hemoglobin levels, red cell distribution width (RDW), white blood cell count, percent segmented neutrophils, percent eosinophils, platelet count, mean platelet volume (MPV), platelet distribution width (PDW), and lactose dehydrogenase (LDH) level were observed among the patient groups. These results are shown in
Table 1.
3. Detection of JAK2 V617F by PCR
DNA from the BM aspirates was isolated by using a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA). A Seeplex JAK2 Genotyping kit (Seegene, Seoul, Korea) or Real-Q JAK2 V617F Detection kit (BioSewoom, Seoul, Korea) was used to detect JAK2 V617F mutations.
4. Screening PCR
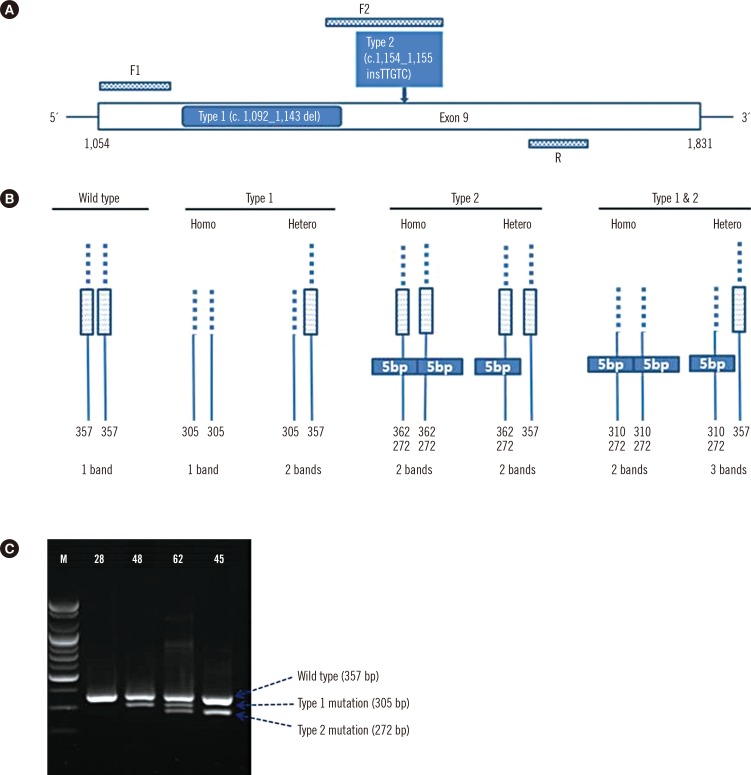
For analysis of
CALR mutations, oligonucleotide primers targeting exon 9 of
CALR were used to amplify the mutation hot spot. PCR primer sets were designed to detect type 1 and type 2 mutations in one reaction, including primers F1 (forward primer 1) 5'-GCA GCA GAG AAA CAA ATG AAG G-3', F2 (forward primer 2) 5'-GCA GAG GAC AAT TGT CGG A-3', and R (reverse primer) 5'-AGA GTG GAG GAG GGG AAC AA-3' (
Fig. 1A, B). Ten nanograms of DNA template, 0.5 µL (10 pmol) of each forward primer, and 1.0 µL (10 pmol) of the reverse primer were added to the PCR premix (20 µL, final volume) (Bioneer, Daejeon, Korea). An initial preheating at 94℃ for 10 min was followed by denaturation at 94℃ for 30 sec, annealing at 64℃ for 30 sec, and extension at 72℃ for 30 sec for 40 cycles followed by a final extension at 72℃ for 7 min. After PCR amplification, gel electrophoresis was performed in a 2% agarose gel at 130 V for 30 min to detect the amplified regions of DNA, and agarose gels were exposed under UV light in a Bio-Rad Gel DOC EZ imager (Bio-Rad, Hercules, CA, USA) (
Fig. 1C). Interpretation was done by comparing bands to the expected product size (wild type
CALR: 357 bp,
CALR type 1 mutation: 302 bp, and
CALR type 2 mutation: 272 bp).
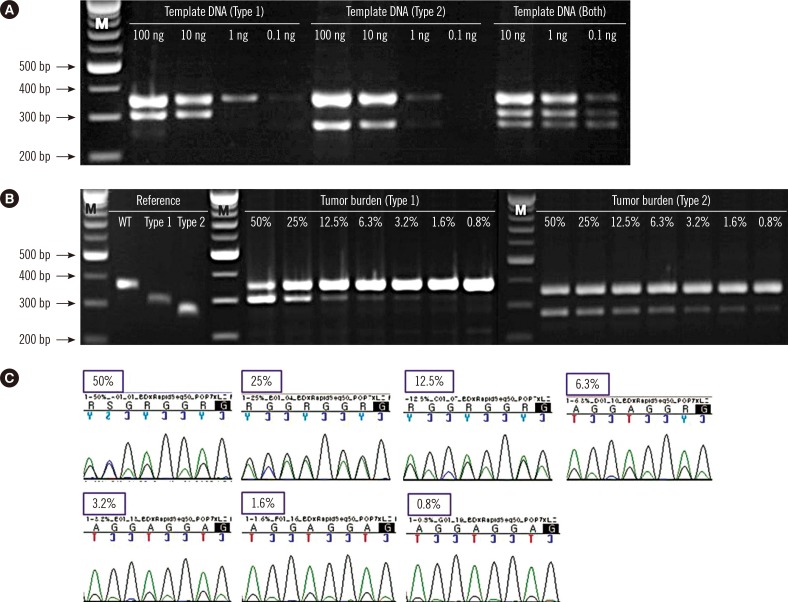
5. Sensitivity of screening PCR in detecting CALR type 1 and type 2 mutations
To study the limit of detection (LoD) of the screening PCR, patient BM DNA carrying
CALR type 1 (No. 48) and type 2 (No. 45) were serially diluted in different concentrations (100 ng, 10 ng, 1 ng, and 0.1 ng). In the case of both mutations (No. 62), three concentrations were tested (10 ng, 1 ng, and 0.1 ng) owing to lack of sample quantity. To determine the assay sensitivity according to tumor burden, the reference sequences for wild-type
CALR and type 1 and type 2
CALR mutants were obtained by gel extraction (GeneAll, Seoul, Korea) from PCR products. The weight of DNA to copy number was calculated (
http://scienceprimer.com/copy-number-calculator-for-realtime-pcr), and the samples were diluted to the same copy number with different tumor burdens (50% mutant, 25% mutant, 12.5% mutant, 6.3% mutant, 3.2% mutant, 1.6% mutant, and 0.8% mutant). Type 1 mixtures were analyzed by Sanger sequencing for a comparison of assay sensitivity.
6. Sanger sequencing
Ten nanograms of DNA template and 1.0 µL (10 pmol) each of primers F1 and R were added to the PCR premix (20 µL final volume) (Bioneer). PCR was performed according to the conditions described above, and then PCR products were purified and sequenced by using the reverse primer and a BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA) on an ABI 3500XL Genetic Analyzer (Applied Biosystems).
7. Fragment analysis
For the fragment analysis, PCR was carried out with 6-FAM-labeled F1 and R primers. PCR products were analyzed by capillary electrophoresis on an ABI 3500XL Genetic Analyzer (Applied Biosystems), followed by fragment analysis using GeneMapper Software 4.1 (Applied Biosystems).
8. Statistical analysis
Sensitivity, specificity, positive predictive value and negative predictive value were calculated by MedCalc software (
https://www.medcalc.org/calc/diagnostic_test.php). Kruskal–Wallis test was used for the analysis of continuous variables, and Fisher's exact test was used for the analysis of categorical variables.
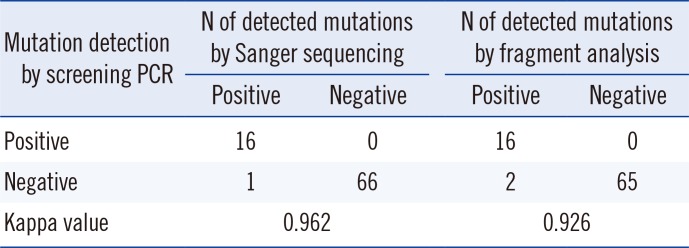
P<0.05 was considered statistically significant. Agreement between results of the detection methods was assessed by using the Kappa statistic (Cohen's kappa coefficient [κ]: <0=poor, 0-0.2=slight, 0.21-0.4=fair, 0.41-0.6=moderate, 0.61-0.8=substantial, and 0.81-1=almost perfect) [
8]. The SPSS 17.0 (SPSS Inc., Chicago, IL, USA) statistical program was used for all calculations.
DISCUSSION
Detection of
CALR mutations in
JAK2 V617F-negative ET and PMF patients has been helpful in diagnosing and predicting the prognosis of patients with previously difficult-to-characterize disease [
4].
CALR mutations have been associated with longer survival times in PMF cases [
9]. Several reports have also suggested that patients with
CALR mutations have a lower incidence of thrombosis and hematologic progression than those with the
JAK2 V617F mutation [
2610].
In many studies,
CALR mutational analysis was performed by PCR followed by Sanger sequencing [
341112]. Some researchers have reported the use of other methods, such as targeted next generation sequencing (NGS) [
13], fragment analysis [
14], and high-resolution melting analysis (HRMA) [
1516]. Sanger sequencing showed a sensitivity of 10% in
CALR type 1 and type 2 mutations; it was less sensitive in samples with a low mutant allele burden [
17]. The maximal reported sensitivity of HRMA in the detection of both
CALR type 1 and type 2 mutations was 2.5% [
17]. Jones et al. [
13] who tested several methods for the detection of
CALR mutations found that the most sensitive method was targeted NGS, which detected mutations down to a 1.25% mutant burden, followed by HRMA at 5%, fragment analysis at 5-10%, and Sanger sequencing at 10-25%. Methods for the detection of acquired mutations within hematologic malignancies showed a range of sensitivities. Here, we showed that our screening PCR can detect type 1 and type 2 mutations in the
CALR gene, since the differential product sizes can be used to discriminate mutants from wild-type alleles.
The screening PCR detected
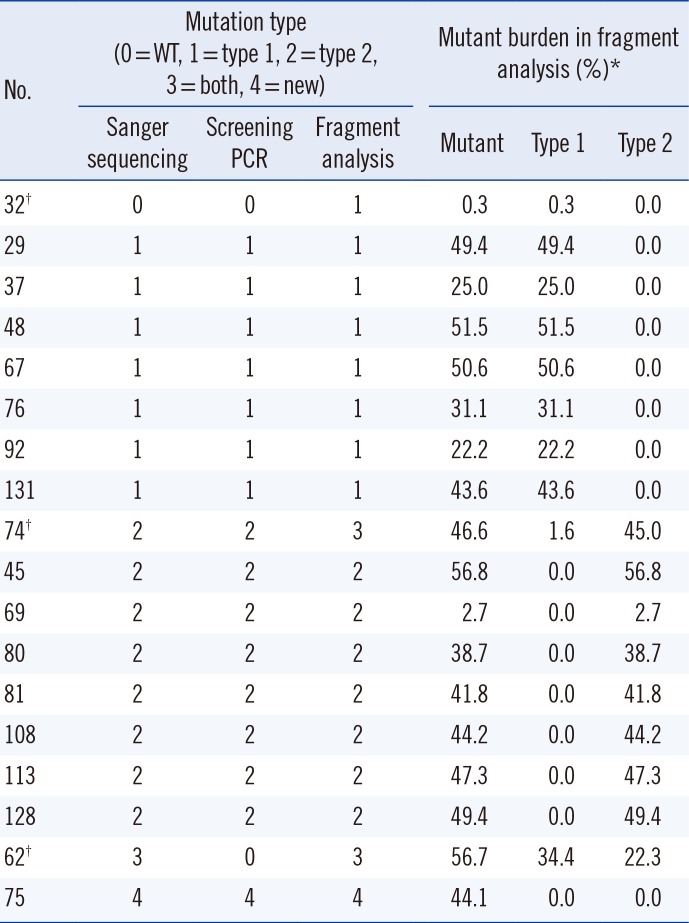
CALR type 1 and type 2 mutants with a maximal sensitivity of 3.2% and <0.8%, respectively, compared with the higher sensitivities obtained by Sanger sequencing (6.3%, type 1 mutation). Because a specific primer for the detection of the type 2 mutation was used in the screening PCR, this system showed higher sensitivity in detecting the type 2 mutation than the type 1 mutation. In this study, the fragment analysis showed greater sensitivity in detecting the type 1 mutation (No. 32 and No. 74) than either Sanger sequencing or the screening PCR (
Table 2). The fragment analysis identified two samples with low type 1 mutant burden (0.3% in No. 32 and 1.6% in No. 74); however, these mutations were not detected by using the other methods. Based on our results, maximal sensitivity in detecting the type 1 mutation was 6.3% by Sanger sequencing, 3.2% by the screening PCR, and <1% by the fragment analysis. The tumor burden of the two samples was too low to detect using Sanger sequencing and the screening PCR. This showed that the screening PCR could result in false negatives in cases with low allele burdens of
CALR mutations. However, no false positive was detected in our screening PCR. Another limitation of the screening PCR is that it cannot detect a single nucleotide substitution or small deletion/insertion like the novel mutation found in one case in this study; however, the frequency of these mutations is rare. Therefore, this screening PCR would be useful as a screening test to detect the major
CALR mutations in laboratories without sequencing equipment.
CALR mutations induce a frameshift through exon 9 deletions or insertions; type 1 and type 2 mutations constitute more than 80% of these mutations [
18]. According to a previous report, at least 36 different indels account for the remaining variants [
413]. In this study, the frequency of
CALR mutations, except for type 1 and type 2 mutations, was markedly low, probably owing to the small sample size. The majority of
CALR mutations are believed to be heterozygous [
4], and all mutations detected in the current study were also heterozygous. The prognostic value or clinical characteristics of zygosity have not yet been studied.
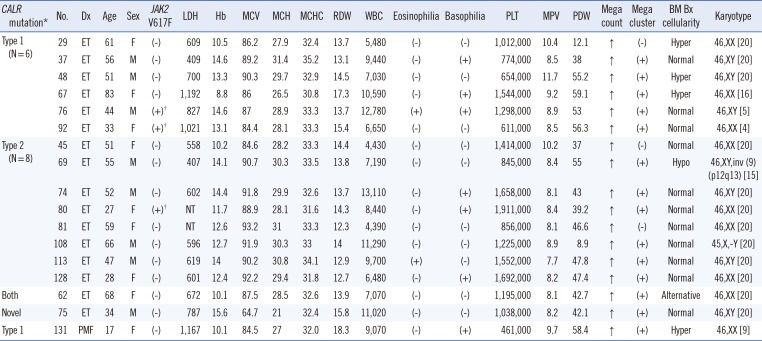
Other researchers have reported a slightly higher frequency of type 1 than type 2 mutation in the ET group (type 1: 45% vs. type 2: 39% [
18] and type 1: 53.6% vs. type 2: 32.1% [
12]). In this study, the frequency of the type 2 mutation (9/16, 56.3%) in ET patients was higher than that of type 1 (6/16, 37.5%). The small sample size of our study may have affected the discordant result with previous reports.
CALR mutations in ET are generally associated with patients of a younger age, male sex, higher platelet count, lower hemoglobin levels, lower leukocyte counts, and lower incidence of thrombotic events. Tefferi et al. [
91819] reported that male sex was associated with only type 1 mutations, and that younger age and higher platelet counts were associated with type 2 mutations. Because of the small sample size, a statistical analysis of
CALR-associated phenotypes was not performed for other disease groups.
Although
JAK2 and
CALR mutations are considered mutually exclusive in patients with ET and PMF, co-expression of these mutations has been identified in a patient with PMF [
20] and a patient with ET [
21]. In the current study, two ET patients (No. 76 and No. 92) had co-existent
JAK2 V617F and type 1
CALR mutations. In another ET patient (No. 80),
JAK2 V617F and type 2
CALR mutations were detected in follow-up BM samples. These findings provide additional exceptions to the rule of mutual exclusiveness of
JAK2 and
CALR mutations. We suggest that an evaluation of
CALR mutations be performed in ET patients with
JAK2 mutations and during follow-up studies. Interestingly, one ET patient (No. 62) had both type 1 and type 2
CALR mutations. This is the first such a case reported in an MPN patient.
In conclusion, the dual negative rate (JAK2 V617F and CALR) was 14.8% in our ET group. In other words, approximately 85% of ET patients were diagnosed by these markers with demonstrated clonality. Our screening PCR is a rapid, sensitive, easy-to-use, and cost-effective method to screen major CALR mutations. We recommend using this rapid screening PCR for the identification of major CALR mutations in suspected MPN patients initially, and then, in the case of a negative result, using a more sensitive method for confirmation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download