Dear Editor,
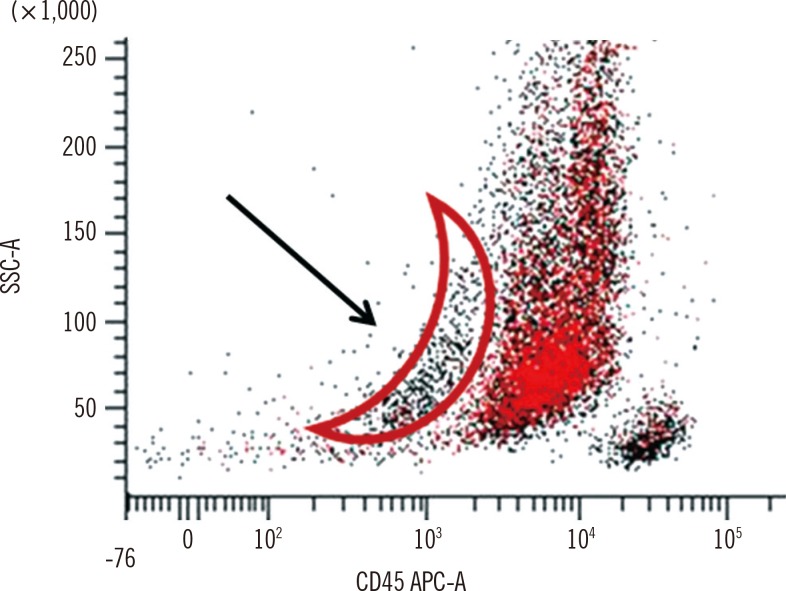
We would like to thank Bennett JM and Wells DA for their insightful comments [1] regarding our manuscript "Lee et al. An unusual case of myeloperoxidase-positive acute megakaryoblastic leukemia. Ann Lab Med 2015;35:466-8" [2]. The case that we reported was diagnostically challenging, and our conclusions were based on various lines of evidence. In particular, the morphological features of blasts were unusual. Most of the blasts were similar to those of acute myeloid leukemia with or without maturation, but some blasts had multiple, clear cytoplasmic projections. In the immunophenotyping study, a similar proportion (approximately 57%) of blasts showed positivity for myeloperoxidase (MPO) and CD41a. We initially considered the possibility of false-positive CD41a expression because of platelet contamination. However, the CD41a-positive population was mainly in the blast population, and not in the platelets, as demonstrated in the backgating analysis (Fig. 1). Accordingly, we concluded that blasts exhibited true positivity of CD41a expression. We have about 25 yr of experience with flow cytometry, including more than 1,000 cases of newly diagnosed acute leukemia. Nonetheless, this was our first observation of blasts that coexpressed MPO and CD41a. We could not find appropriate diagnostic criteria for this case. Considering previously reported cases, we concluded that this case was MPO-positive acute megakaryoblastic leukemia (AMKL).
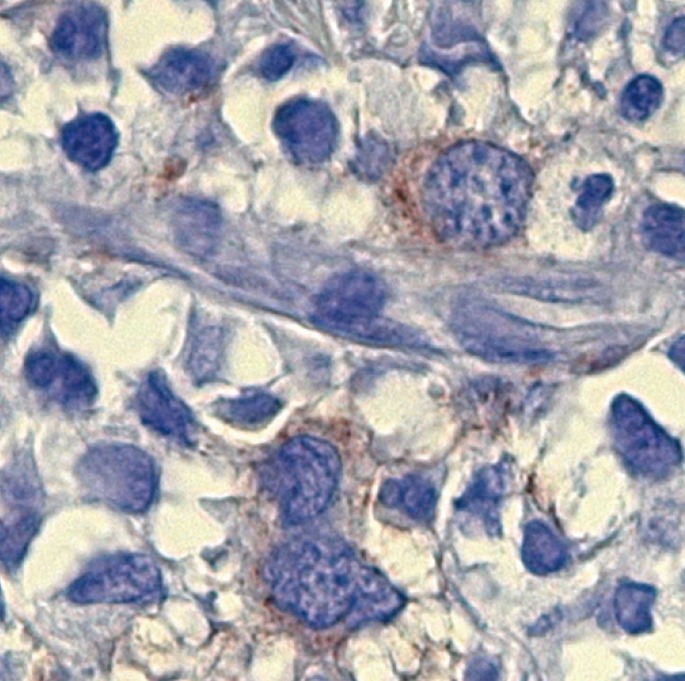
We were unable to perform flow cytometry for CD61 or cytospin immunofluorescence, but we performed additional CD61 immunohistochemical stains (IHC). The CD61-positive blasts represented approximately 3% of all blasts in the biopsy (Fig. 2). Unlike bone marrow aspirates, the blasts were infrequently detected in the biopsy section. We believe the sensitivity of flow cytometry is superior to that of IHC. It is also possible that malignant cells show a discrepancy in the expression of lineage markers.
There was no fibrosis in our case. A reticulin stain was negative, and a test for the JAK2 V617F mutation was negative. HemaVision (DNA Technology, Aarhus, Denmark) did not reveal any genetic abnormalities.
Tallman et al. [3] reviewed previously diagnosed AMKL and found two MPO-positive cases. Their diagnosis mainly relied on morphology, and there might be inconsistencies when considering current techniques. One patient had FVIII (+) and t(3;3), supporting AMKL. The other patient had no platelet antigen, a normal karyotype, and was positive for the monocytic antigen. The authors concluded that these cases were diagnostic controversies. Despite their reliance on morphologic evidence, we used that report as a reference. In the case of Majhi et al. [4], the possibility of leukemic transformation of myelofibrosis was not mentioned. Pediatric myelofibrosis is rare [5], and the disease progression to leukemia is extremely rare, unlike in adults [6]. Moreover, it is difficult to distinguish between AMKL and acute myelofibrosis because some AMKL accompany extensive bone marrow fibrosis, like that observed in a patient [7]. Therefore, we could not completely agree with that opinion.
We could not explain the pathophysiology of this case, but we believe it is important to introduce this unusual phenotype in AMKL for further research.
References
1. Bennett JM, Wells DA. Re: Lee H, et al. An unusual case of myeloperoxidase-positive acute megakaryoblastic leukemia. Ann Lab Med 2015;35:466-8. Ann Lab Med. 2015; 35:542–543. PMID: 26206694.

2. Lee H, Kim Y, Kim YJ, Han K. An unusual case of myeloperoxidase-positive acute mgakaryoblastic leukemia. Ann Lab Med. 2015; 35:466–468. PMID: 26131422.
3. Tallman MS, Neuberg D, Bennett JM, Francois CJ, Paietta E, Wiernik PH, et al. Acute megakaryocytic leukemia: the Eastern Cooperative Oncology Group experience. Blood. 2000; 96:2405–2411. PMID: 11001891.
4. Majhi U, Murhekar K, Sundersingh S, Rajalekshmi KR. Megakaryoblastic leukemia presenting as pancytopenia and extensive myelofibrosis in a child diagnosed by myeloid markers and CD 31. Indian J Med Paediatr Oncol. 2012; 33:59–61. PMID: 22754213.

5. Barbui T. How to manage children and young adults with myeloproliferative neoplasms. Leukemia. 2012; 26:1452–1457. PMID: 22252311.

6. DeLario MR, Sheehan AM, Ataya R, Bertuch AA, Vega C 2nd, Webb CR, et al. Clinical, histopathologic, and genetic features of pediatric primary myelofibrosis-an entity different from adults. Am J Hematol. 2012; 87:461–464. PMID: 22389089.

7. Arber DA, Brunning RD, Orazi A, Porwit A, Peterson L, Thiele J, et al. Acute myeloid leukemia, not otherwise specified. In : Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC;2008. p. 130–139.
Fig. 1
The backgating of CD41a shown in the original article [2]. Most platelets are located in the area marked with an arrow, forming an arch pattern. CD41a expression was mainly detected in the blast population, not in the platelet area.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download