Dear Editor
Campylobacter are curved, gram-negative, non-spore-forming rods that usually grow in a microaerophilic environment. To date, 22 species have been assigned to Campylobacter. Although most Campylobacter species are zoonotic, C. jejuni, C. coli, and C. fetus are well-known human pathogens; C. upsaliensis and C. lari are also considered enteropathogenic bacteria [1].
C. hyointestinalis was first isolated from pigs with proliferative enteritis in 1983 [2] and was initially considered a pathogen of pigs and rodents; however, there have been two previous reports of isolation from patients with proctitis and diarrhea [34]. C. hyointestinalis is now thought to be a human pathogen, but reported cases are rare [134]. We report a case of gastroenteritis possibly induced by C. hyointestinalis, which was identified in stool specimens and confirmed with 16S ribosomal RNA (rRNA) gene sequencing, biochemical testing, and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS).
The patient was an 88-yr-old male who visited the emergency room because of right-side weakness. He had a history of left middle cerebral artery infarction and rectal cancer and underwent low anterior resection with coloanal anastomosis and postoperative adjuvant radiotherapy five years earlier. He was diagnosed as having recurrent left middle cerebral artery infarction and admitted for antithrombotic therapy. On day three after admission, he complained of diarrhea, which was unresponsive to antidiarrheal agents. Physical examination showed mild hypoesthesia of the right extremities unrelated to the diarrhea. Laboratory investigation showed hemoglobin level of 13.0 g/dL, leukocyte count of 5.58×109/L, platelet count of 270×109/L, C-reactive protein level of 1.6 mg/L, and erythrocyte sedimentation rate of 9 mm/hr.
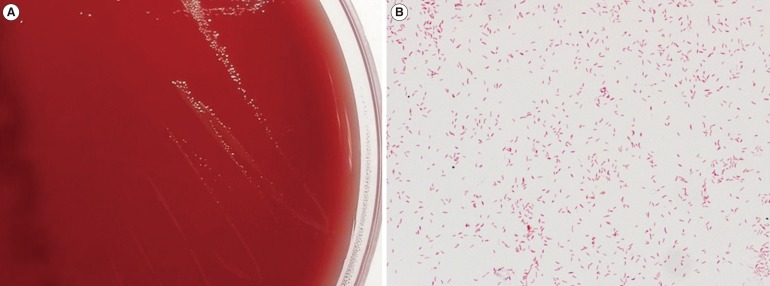
Stool culture was performed consecutively on days three and four. No remarkable growth of enteropathogenic bacteria was observed on MacConkey agar or salmonella-shigella agar. A large number of gray colonies positive for oxidase and catalase (Fig. 1A) were observed in a selective medium for Campylobacter species under microaerophilic conditions below 42℃ [5]. Curved gram-negative rods were observed on the smear preparation (Fig. 1B). The hippurate test was negative, and H2S production was positive in triple sugar iron (TSI) agar. Species identification performed with a Bruker Microflex MALDI-TOF MS system (Bruker Daltonics, Bremen, Germany) showed C. hyointestinalis in both stool cultures (day 3 score: 2.199; day 4 score: 2.159). For confirmation, the 605 base pairs of the 16S rRNA gene were sequenced, and the ezTaxon database (http://www.ezbiocloud.net/eztaxon) showed 99.5% identity with GenBank sequence JHQP01000019 (C. hyointestinalis) and 99.3% identity with GenBank sequence AY621303 (C. fetus). These results confirmed the isolate as C. hyointestinalis (C. fetus: negative for H2S production in TSI agar). However, an examination for parasites and ova and tests for Clostridium difficile were negative, including toxigenic culture and toxin PCR assay using the GeneXpert system (Cepheid, Sunnyvale, CA, USA).
Antimicrobial susceptibility testing (AST) was performed via the disk diffusion method on blood agar incubated microaerobically for 24 hr. Zone diameters of ciprofloxacin and erythromycin were 33 mm and 36 mm, respectively, which suggested susceptibility according to the interpretive criteria of the Clinical and Laboratory Standard Institute guidelines for C. jejuni and C. coli. [6]. The patient was discharged without antibiotic treatment on day six. After two weeks, he returned to the infectious diseases outpatient clinic owing to persistent diarrhea with hematochezia. Oral ciprofloxacin (500 mg daily) for 14 days was prescribed without further examination. The patient did not return to the clinic; therefore, additional follow-up was not performed.
The clinical significance of C. hyointestinalis has not been clearly established. However, previous reports [34] suggest that it is a human pathogen causing proctitis and enteritis. Although other pathogens for diarrhea including enterohemorrhagic Escherichia coli, Yersinia, rotavirus, norovirus, and enteric adenovirus were not considered, C. hyointestinalis was the only pathogen identified in this case. This species was isolated from two consecutive stool cultures, which indicate C. hyointestinalis as a possible pathogen of diarrhea. Furthermore, this isolate was initially identified as C. hyointestinalis with MALDI-TOF MS. Because identification with conventional biochemical methods including the hippurate test, H2S production, and growth under specific conditions is cumbersome and time-consuming, MALDI-TOF MS may be a rapid and accurate method for identifying Campylobacter species [7].
In conclusion, we isolated C. hyointestinalis from a patient stool specimen for the first time in Korea and confirmed its identification with MALDI-TOF MS and 16S rRNA gene sequencing. This isolate appeared to be a pathogenic cause for enteritis.
References
1. Fitzgerald C, Nachamkin I. Camphylobacter and Arcobacter. Manual of clinical microbiology. 10th ed. Washington DC: ASM press;2011. p. 885–899.
2. Gebart CJ, Ward GE, Chang K, Kurtz HJ. Campylobacter hyointestinalis (new species) isolated from swine with lesions of proliferative ileitis. Am J Vet Res. 1983; 44:361–367. PMID: 6838031.
3. Fennell CL, Rompalo AM, Totten PA, Bruch KL, Flores BM, Stamm WE. Isolation of "Campylobacter hyointestinalis" from a human. J Clin Microbiol. 1986; 24:146–148. PMID: 3722361.
4. Edmonds P, Patton CM, Griffin PM, Barrett TJ, Schmid GP, Baker CN, et al. Campylobacter hyointestinalis associated with human gastrointestinal disease in the United States. J Clin Microbiol. 1987; 25:685–691. PMID: 3571477.
5. Chung Y, Lee K, et al. Diagnostic Microbiology. 5th ed. Seoul: Seoheung;2014. p. 374–385.
6. Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline. M45-A2. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2010.
7. Alispahic M, Hummel K, Jandreski-Cvetkovic D, Nöbauer K, Razzazi-Fazeli E, Hess M, et al. Species-specific identification and differentiation of Arcobacter, Helicobacter and Campylobacter by full-spectral matrix-associated laser desorption/ionization time of flight mass spectrometry analysis. J Med Microbiol. 2010; 59:295–301. PMID: 19959629.
Fig. 1
Colony and microscopic characteristics of Campylobacter hyointestinalis. (A) Gray, flat, irregular and spreading colonies were observed on a blood agar plate after 48 hr of microaerobic incubation. (B) Curved gram-negative rods from a smear of colonies obtained from the blood agar plate (× 1,000).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download