Abstract
Quality control (QC) processes are being performed in the majority of clinical microbiology laboratories to ensure the performance of microbial identification and antimicrobial susceptibility testing by using ATCC strains. To obtain these ATCC strains, some inconveniences are encountered concerning the purchase cost of the strains and the shipping time required. This study was focused on constructing a database of reference strains for QC processes using domestic bacterial strains, concentrating primarily on antimicrobial susceptibility testing. Three strains (Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus) that showed legible results in preliminary testing were selected. The minimal inhibitory concentrations (MICs) and zone diameters (ZDs) of eight antimicrobials for each strain were determined according to the CLSI M23. All resulting MIC and ZD ranges included at least 95% of the data. The ZD QC ranges obtained by using the CLSI method were less than 12 mm, and the MIC QC ranges extended no more than five dilutions. This study is a preliminary attempt to construct a bank of Korean QC strains. With further studies, a positive outcome toward cost and time reduction can be anticipated.
To ensure test performance, microbial identification and performing an antimicrobial susceptibility test (AST) require testing of standard quality control (QC) strains in clinical diagnostic microbiology laboratories [123]. Most clinical microbiology laboratories conduct periodic internal QC tests using QC strains, which have established reproducibility and the QC ranges for regularly used antibiotics. The selection process for a QC strain is sophisticated, especially for those of the AST QC strain that requires multiple considerations such as antibiotics, susceptibility testing methods, and QC ranges. The ATCC strains, which are typically shipped from the US, are commonly used as the QC strain in microbiology laboratories. The financial burden for purchasing and time required for delivery of the ordered strains pose inconveniences to these laboratories. Although organizations such as the National Culture Collection of Pathogens (NCCP) in Korea are preserving many bacterial strains, these strains are rarely utilized in the clinical microbiology laboratories, since their antimicrobial susceptibility data are unavailable. Hence, the objective of this study was to obtain preliminary AST QC ranges using bacteria preserved in the NCCP with a final goal of establishing a reference bank using local bacterial strains.
In the tier 1 step, three strains (Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus), which are the most commonly used for QC in clinical microbiology laboratories, were selected as target strains. Several isolates of each of the three strains, preserved in the NCCP in Korea, were evaluated with multiple passages and multiple freeze/thaw cycles. Disk diffusion tests were performed according to the CLSI M23 [2] by using the group A and B antibiotics suggested in the CLSI M100 [4]. The selected strains (E. coli NCCP 13894, P. aeruginosa NCCP 12297, and S. aureus NCCP 11486), which exhibited the most stable and legible results, were selected because they satisfied the following conditions: reproducibility and stability through multiple passages, according to the CLSI M23 [2].
The tier 2 steps were also performed following the guidelines in the CLSI M23 [2] with modification to the number of locations for which AST was conducted. To prepare inoculum suspensions, fresh-grown strains selected in tier 1 testing were sent to the following seven independent laboratories in Korea: CHA Bundang Medical Center (Seongnam), Kangnam Sacred Heart Hospital (Seoul), Myongji Hospital (Goyang), National Health and Insurance Corporation Ilsan Hospital (Goyang), Neodin Medical Institute (Seoul), Severance Hospital (Seoul), and Yongin Severance Hospital (Yongin). In each laboratory, inocula of each of the three strains were prepared to a turbidity of 0.5 McFarland standards and collected in the laboratory of Severance Hospital. Each inoculum suspension was then tested by using ten replicates of each of the selected strains on three dates, each with three separate media lots and two separate disk lots. The three media lots of Mueller-Hinton agar used for ASTs were manufactured by the following companies: BD Diagnostics (Sparks, MD, USA), Oxoid (Basingstoke, United Kingdom), and Bio-Rad (Mames-la-Conquette, France). The disk lots used for disk diffusion were manufactured by BD Diagnostics and Oxoid, and antibiotics were purchased from Sigma Chemical Company (St. Louis, MO, USA). Eight antibiotics, that is, piperacillin, ceftazidime, cefepime, aztreonam, imipenem, meropenem, amikacin, and gentamicin, were tested for E. coli and P. aeruginosa. For S. aureus, the antibiotics penicillin, cefoxitin, vancomycin, teicoplanin, clindamycin, erythromycin, tetracycline, and ciprofloxacin were tested. Agar dilution and disk diffusion tests were conducted according to the CLSI M7 [5] and the CLSI M2 [6], respectively. Proposed QC ranges of minimal inhibitory concentrations (MICs) and zone diameters (ZDs) were obtained on the basis of the CLSI M23 guideline by using means±standard deviations with adjustment to include at least 95% of the observed results [2]. The Range Finder method encompassing 95% of the value, that is, from the lower 2.5% of the distribution to the upper 97.5% of the value, was applied alternatively for comparison of the QC ranges calculated using the CLSI method [1].
The proposed ranges of MIC (µg/mL) and ZD (mm) calculated for the selected E. coli are summarized in Table 1. For all tested antibiotics, the ranges of MIC calculated using both the CLSI and Range Finder methods fell within three twofold dilution ranges, and 100% of all MIC results were within the calculated QC ranges. The ranges of proposed ZD ranges calculated using the CLSI and Range Finder methods were 6-11 mm and 9-15 mm, respectively [piperacillin (9; 11), ceftazidime (8; 14), cefepime (10; 13), aztreonam (9; 14), imipenem (8; 13), meropenem (9; 12), amikacin (6; 9), and gentamicin (9; 13)], and 95.2-96.4% and 96.6-98.6% of ZD results were within the QC ranges.
The proposed ranges of MIC and ZD for the selected P. aeruginosa are shown in Table 2. For all tested antibiotics, the proposed ranges of MIC obtained by both the CLSI and Range Finder methods were within three twofold dilution MIC ranges, and 98.3-100% of all MIC results were within the calculated QC ranges. The ranges of proposed ZD ranges calculated using the CLSI and Range Finder methods were 5-9 mm and 8-14 mm, respectively, and 95.2-96.4% and 96.6-98.6% of ZD results were within the proposed QC ranges.
The range of MIC and ZD calculated for the selected S. aureus are summarized as proposed ranges in Table 3. For all tested antibiotics, 100% of the MIC results calculated by both the CLSI and Range Finder methods fell within the MIC ranges within three twofold dilution MIC ranges, except those of teicoplanin that was calculated by the CLSI method within four twofold ranges. The range of proposed ZD ranges calculated using the CLSI and Range Finder methods were 5-11 mm and 9-15 mm, respectively, and 95.2-96.4% and 95.2-98.6% of results were within the proposed ZD ranges.
Reliable and reproducible ASTs for clinically isolated microorganisms are necessary to provide physicians with critical information leading to the appropriate use of antimicrobials [78]. To guarantee the results of ASTs, routine QC testing using QC strains is essential in clinical microbiology [29]. When selecting the QC strain and determining the QC ranges, caution is required for the reproducibility of test results, performance for the objectives of using the QC strains, stability of the strain, etc. [2]. Although there are no consensus criteria concerning the ideal QC range of MIC and ZD for ASTs, excessively broad ranges of ZD and MIC are not recommended: a ZD range of >12 and twofold dilution rage of ≥5 for MIC [1]. The QC ranges deducted by our study satisfied this general concept: the ranges of MICs were within four twofold dilution MIC ranges, and the ranges of ZD calculated using the CLSI method were less than 12 mm.
Limitations of this study include subculturing of the three strains and performing ASTs using the agar dilution method and disk diffusion method; furthermore, the results were interpreted in a laboratory, and the number of antimicrobials tested was limited to eight. Thus, the QC strains and obtained QC ranges are inadequate for immediate adoption by clinical microbiology laboratories owing to a possible bias of QC ranges and a lack of validation using automated microbroth dilution methods. However, standardized inoculum suspensions for the agar dilution method and disk diffusion test were prepared in seven independent laboratories to reflect the inter-laboratory variations and were transported in cold storage within two hours to prevent bacterial overgrowth.
In conclusion, this study is the first attempt to establish QC ranges for MIC and ZD of antimicrobial agents in Korea. The selected QC strains show positive results for constructing a bank of QC strains for domestic clinical microbiology laboratories, though the strain and calculated QC ranges are inapplicable because of the limitations of this study. With further studies, a positive outcome toward cost and time reduction can be anticipated.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Korea (2012-E45004-00) (A110567).
References
1. Turnidge J, Bordash G. Statistical methods for establishing quality control ranges for antibacterial agents in Clinical and Laboratory Standards Institute susceptibility testing. Antimicrob Agents Chemother. 2007; 51:2483–2488. PMID: 17438045.

2. Clinical and Laboratory Standards Institute. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline-third ed. M23-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2008.
3. Gavan TL, Jones RN, Barry AL, Fuchs PC, Gerlach EH, Matsen JM, et al. Quality control limits for ampicillin, carbenicillin, mezlocillin, and piperacillin disk diffusion susceptibility tests: a collaborative study. J Clin Microbiol. 1981; 14:67–72. PMID: 6455443.

4. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-second informational supplement, M100-S22. Wayne, PA: Clinical Laboratory Standards Institute;2012.
5. Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-eighth ed. M07-A8. Wayne, PA: Clinical and Laboratory Standards Institute;2009.
6. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests; approved standard-tenth ed. M02-A10. Wayne, PA: Clinical and Laboratory Standards Institute;2009.
7. Kuper KM, Boles DM, Mohr JF, Wanger A. Antimicrobial susceptibility testing: a primer for clinicians. Pharmacotherapy. 2009; 29:1326–1343. PMID: 19857149.

8. Shin SY, Koo SH, Kwon KC, Park JW, Ko CS, Song JH, et al. Evaluation of the Vitek 2 Korean Antimicrobial Susceptibility Testing Cards AST N056 and AST N055. Korean J Clin Microbiol. 2008; 11:23–28.

9. Njoroge SW. Risk management in the clinical laboratory. Ann Lab Med. 2014; 34:274–278. PMID: 24982831.

Table 1
Proposed quality control ranges of antimicrobial agents for selected Escherichia coli using disk diffusion and agar dilution tests
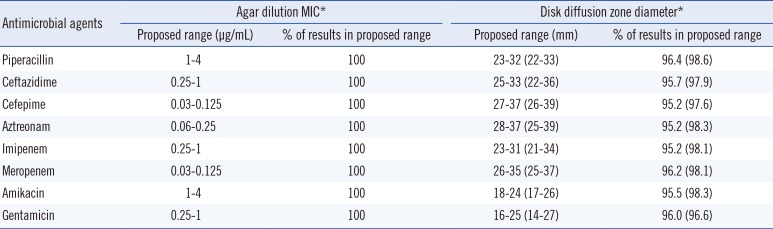
*Range Finder results, if different, are in parentheses [1].
Abbreviation: MIC, minimal inhibitory concentration.
Table 2
Proposed quality control ranges of antimicrobial agents for selected Pseudomonas aeruginosa using disk diffusion and agar dilution tests
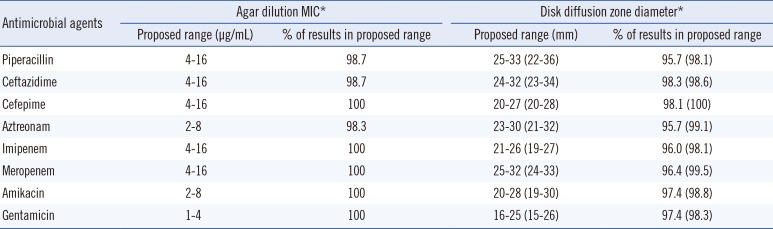
*Range Finder results, if different, are in parentheses [1].
Abbreviation: MIC, minimal inhibitory concentration.
Table 3
Proposed quality control ranges of antimicrobial agents for selected Staphylococcus aureus disk diffusion and agar dilution tests
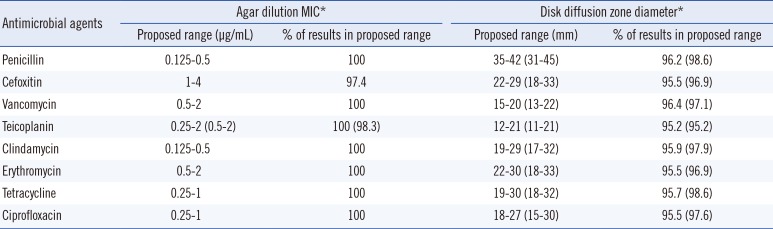
*Range Finder results, if different, are in parentheses [1].
Abbreviation: MIC, minimal inhibitory concentration.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download