Abstract
Background
Conventional screening for congenital adrenal hyperplasia (CAH) using immunoassays generates a large number of false-positive results. A more specific liquid chromatography-tandem mass spectrometry (LC-MS/MS) method has been introduced to minimize unnecessary follow-ups. However, because of limited data on its use in the Korean population, LC-MS/MS has not yet been incorporated into newborn screening programs in this region. The present study aims to develop and validate an LC-MS/MS method for the simultaneous determination of seven steroids in dried blood spots (DBS) for CAH screening, and to define age-specific reference intervals in the Korean population.
Methods
We developed and validated an LC-MS/MS method to determine the reference intervals of cortisol, 17-hydroxyprogesterone, 11-deoxycortisol, 21-deoxycortisol, androstenedione, corticosterone, and 11-deoxycorticosterone simultaneously in 453 DBS samples. The samples were from Korean subjects stratified by age group (78 full-term neonates, 76 premature neonates, 89 children, and 100 adults).
Results
The accuracy, precision, matrix effects, and extraction recovery were satisfactory for all the steroids at three concentrations; values of intra- and inter-day precision coefficients of variance, bias, and recovery were 0.7-7.7%, -1.5-9.8%, and 49.3-97.5%, respectively. The linearity range was 1-100 ng/mL for cortisol and 0.5-50 ng/mL for other steroids (R2>0.99). The reference intervals were in agreement with the previous reports.
Congenital adrenal hyperplasia (CAH), the most common adrenal gland disorder in infants and children, is a group of autosomal recessive disorders of adrenal cortisol biosynthesis [12]. In Caucasians, 21-hydroxylase deficiency, the classical form of CAH, accounts for more than 90% of all cases, whereas 5% are caused by 11-hydroxylase deficiency [34]. The prevalence is approximately 1 in 15,000 to 16,000 live births [5].
At present, immunoassays are commonly used for the measurement of 17-hydroxyprogesterone in CAH screening. Although widely used in laboratories and hospitals because of its simplicity, speed, and sensitivity, the reliability of the method has been questioned [67]. Immunoassays produce a large number of false-positive results especially in preterm or acutely ill babies, which is attributable to the delayed physiological maturation of 11-hydroxylase, poor kidney function, and elevated 17-hydroxyprogesterone levels due to illness and stress [89]. Furthermore, cross-reactivity with other endogenous steroids poses additional problems [67]. This limitation creates a significant burden on follow-up programs as well as stress on the families of patients [1011].
Recently, liquid chromatography-tandem mass spectrometry (LC-MS/MS) has emerged as the most accurate method for measuring small molecules [12]. In addition to high specificity in complex sample matrices, LC-MS/MS can measure several steroid hormones simultaneously [13]. Steroid profiling with LC-MS/MS allows the evaluation of the status of the enzymes involved in the adrenal steroid biosynthetic pathways, thus, it is a better diagnostic tool than the evaluation of a single steroid.
In Korea, the prevalence of CAH was estimated to be 1 in 39,069 live births in 2013 [14]. Since 2006, CAH has been routinely screened as a part of the newborn screening program in Korea, with 17-hydroxyprogesterone as the target analyte, which addresses the most common type of CAH [1015]. However, there are few laboratories where LC-MS/MS, a more specific analytical method, is used as the primary screening method. Hence, LC-MS/MS data for the Korean population are limited. In this study, we developed and validated a method that accurately detects cortisol, 17-hydroxyprogesterone, 11-deoxycortisol, 21-deoxycortisol, androstenedione, corticosterone, and 11-deoxycorticosterone in dried blood spots (DBS) and is suitable for the screening and diagnosis of CAH in neonates. Since the reference interval is method-specific, we also determined age-specific reference intervals for the seven aforementioned steroids for healthy Korean neonates and adults for the clinical use of LC-MS/MS.
Cortisol, 17-hydroxyprogesterone, 11-deoxycortisol, 21-deoxycortisol, androstenedione, and corticosterone were purchased from Sigma-Aldrich (St. Louis, MO, USA), and 11-deoxycorticosterone was obtained from Clearsynth Labs (Clearsynth Labs Ltd., Mumbai, India). Deuterated standards d4-cortisol, d8-17-hydroxyprogesterone, d2-11-deoxycortisol, d8-21-deoxycortisol, d7-androstenedione, d8-corticosterone, and d8-11-deoxycorticosterone were obtained from C/D/N Isotopes Inc. (Pointe-Claire, QC, Canada). Further, steroid-free serum was purchased from Golden West Biologicals Inc. (Temecula, CA, USA). All solvents (methanol, acetonitrile, and water) were of HPLC grade and were obtained from Burdick & Jackson (Honeywell International, NJ, USA). Blood was collected on filter cards type S&S 903 from Whatman Schleicher & Schuell (Dassel, Germany).
Analyses were performed on an Agilent 6490 triple quadrupole mass spectrometer equipped with an Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, CA, USA). The column used was a Kinetex 2.6 µm XB-C18 (2.1 mm×50 mm) (Phenomenex, Torrance, CA, USA) that was maintained at 40℃. Mobile phases consisted of water (mobile A) and methanol (mobile B). The flow rate was 0.3 mL/min, and the final injection volume of each sample was 20 µL. Quantitative analyses were performed in multiple reaction-monitoring (MRM) mode. All acquisition methods used the following parameters: capillary voltage of 3,500 V, nozzle voltage of 500 V, sheath gas flow of 11 L/min (ultra high purity [UHP] nitrogen) at 400℃, drying gas flow of 12 L/min at 290℃, and nebulizer gas flow of 50 psi. Other operating conditions for the MS are shown in Table 1.
Two blood spots, each 3 mm in diameter and tantamount to 3.1 µL of whole blood, were punched into microliter plates, and 200 µL of the extraction buffer (50% methanol + 50% acetonitrile + internal standard at a final concentration of 0.5 ng/mL) was added. After repeating this step twice, 400 µL of this solution was incubated in a thermoshaker for 1 hr at 750 rpm and 37℃. After drying in a concentrator (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) for 90 min, the extract was reconstituted with 30 µL of 10% methanol and centrifuged for 5 min at 13,000 rpm. Twenty microliters of the supernatant was then injected into the HPLC column.
DBS calibrators, quality controls, and DBS for evaluating extraction recovery and matrix effects were prepared from the venous blood samples by using a modified method reported by Lacey et al. [16]: red blood cells were washed thrice with saline, and then diluted with steroid-free serum to obtain a hematocrit of 55%.
The seven steroids and the deuterated internal standards were dissolved in acetonitrile/water at a dilution of 70:30 (v/v) to obtain a final concentration of 1 mg/mL. This was further diluted in methanol/water at 10:90 (v/v) at a final concentration of 1 µg/mL and stored at -70℃. These stock solutions were added to steroid-free blood to obtain calibrators at final concentrations of 0 (blank), 1, 5, 25, 50, and 100 ng/mL for cortisol, and 0, 0.5, 2.5, 12.5, 25, and 50 ng/mL for the other steroids. For controls, stock solutions of each steroid were spiked into steroid-free blood to achieve final concentrations of 2.5, 10, and 75 ng/mL for cortisol, and 1.25, 5, and 37.5 ng/mL for the other steroids. Subsequently, calibrators and controls were spotted on filter paper cards, dried at room temperature for more than 12 hr, and stored at -70℃.
The intra- and inter-day precision and accuracy were determined by analyzing five replicates at three concentrations (2.5, 10, and 75 ng/mL for cortisol, and 1.25, 5, and 37.5 ng/mL for the other six steroids) for five independent assay runs. Linearity was evaluated by using calibrators containing steroid concentrations between 0.5 and 50 ng/mL, and cortisol concentrations from 1-100 ng/mL. The lower limit of detection was defined as the lowest concentration with a signal-to-noise ratio >5:1. Meanwhile, the lower limit of quantification was defined as the lowest concentration with a signal-to-noise ratio >10:1 and a precision of CV <20% from analysis of five replicates. Extraction recovery and matrix effects were investigated by adopting the principles suggested by Matuszewski et al. [17]. The analytes were spiked prior to and after extraction (pre- and post-extracted sets, respectively) and added to a "blank" reconstitution solvent (reference). The extraction recovery was determined at two different concentrations (10 and 75 ng/mL for cortisol, and 5 and 37.5 ng/mL for the other steroids) in triplicate. Further, the extraction recovery was calculated as the ratio of the mean peak area of an analyte spiked before extraction to the mean peak area of an analyte spiked after extraction, multiplied by 100. Meanwhile, the matrix effects were calculated as the ratio of the mean peak area of an analyte spiked after extraction to the mean peak area of an analyte spiked into blank solvent, multiplied by 100. Values >100% and <100% indicate ionization enhancement and suppression, respectively. In addition, a DBS sample from a patient with 21-hydroxylase deficiency was analyzed.
To determine the reference intervals for the seven steroids in Korean subjects, a total of 154, 89, and 100 DBS samples from neonates (76 premature, 78 full-term neonates), children, and adults, respectively, were analyzed by using the LC-MS/MS protocol. Samples were collected from January 2012 to March 2014. For neonates, whole blood was collected in DBS cards. For children and adults, venous blood was collected into a EDTA tube, and a 25-µL drop of whole blood (the optimal volume recommended by the card manufacturer) was applied to a Whatman 903 card. The reference interval was determined according to the CLSI guideline [18]. Since our intention was comparing the reference intervals to already reported reference intervals rather than establishing new reference intervals, we conducted experiments with insufficient number of samples recommended by CLSI.
The results were compared to those of previously published reports in other populations. This study protocol was approved by the institutional review board of Samsung Medical Center (IRB No. 2012-08-058).
A total ion chromatogram and representative ion-pair LC-MS/MS chromatograms are shown in Fig. 1. Analyses of steroid-free samples showed no interfering peaks at the retention times of the steroids or internal standards. The run time for each sample was 20 min.
The assay performance is summarized in Table 2. The accuracy and precision were satisfactory at all the concentrations tested. Values of intra- and inter-day precision coefficients of variance, bias, and recovery were 0.7-7.7%, -1.5-9.8%, and 49.3-97.5%, respectively. The linear assay range was observed to be 1.0-100 ng/mL for cortisol, and 0.5-50.0 ng/mL for the other steroids (R2>0.99). The lower limit of detection was 0.1 ng/mL for cortisol and 11-deoxycorticosterone, 0.2 ng/mL for androstenedione and 11-deoxycortisol, and 0.3 ng/mL for 17-hydroxyprogesterone, corticosterone, and 21-deoxycortisol. Meanwhile, the lower limit of quantification was 1.0 ng/mL for cortisol and 0.5 ng/mL for the other steroids, with CVs within 20%. The extraction recovery was consistent across the concentration levels. It was 49.3-62.3% for 11-deoxycortisol and 69.4-97.5% for the other steroids. Moreover, there was no significant ionization suppression or enhancement. Further, the matrix effects ranged from 80.1-80.5% for androstenedione and 11-deoxycortisol and 90.5-100.4% for the other steroids. Analysis of a sample from a patient with 21-hydroxylase deficiency showed agreeable results and were as follows: cortisol, 6.25 ng/mL; 17-hydroxyprogesterone, >50 ng/mL; 11-deoxycortisol, 0.37 ng/mL; 21-deoxycortisol, 38.60 ng/mL; androstenedione, 3.10 ng/mL; corticosterone, 1.09 ng/mL; and 11-deoxycorticosterone, 31.10 ng/mL.
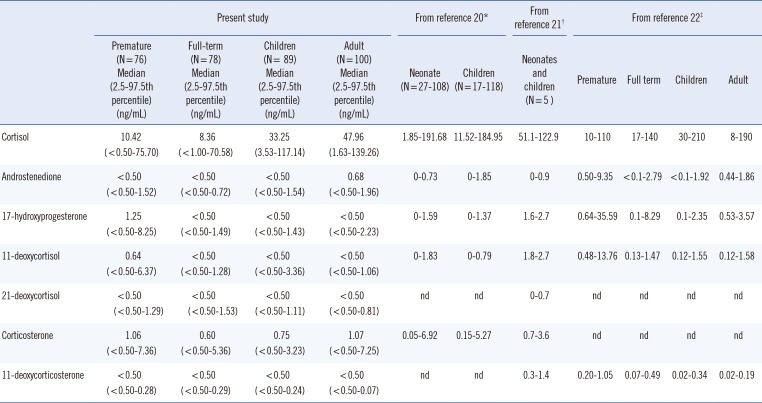
The comparison of reference intervals and median values of the seven steroids determined in this study with those reported by other studies is summarized in Table 3.
In this study, an LC-MS/MS method for the simultaneous quantification of seven steroids in DBS was validated. In addition, we determined age-specific reference intervals of the seven steroids in the Korean population.
The method presented here allows for the simultaneous and rapid quantification of the steroids cortisol, 17-hydroxyprogesterone, 11-deoxycortisol, 21-deoxycortisol, androstenedione, corticosterone, and 11-deoxycorticosterone with high specificity. Current immunoassays generate large number of false-positive results, with low positive predictive value [1119]. To reduce the number of unnecessary tests, anxiety to families and physicians, and burden to the newborn screening follow-up program, quantification of 17-hydroxyprogesterone by a highly specific LC-MS/MS method can be used as a primary or secondary test. For example, 84% of the false-positive results were eliminated and the follow-up duration was significantly reduced from 75 to 8 days when the LC-MS/MS method was applied [11].
Furthermore, by using steroid profiling a subtle differential diagnosis between subtypes is possible [2021]. The enzyme 21-hydroxylase catalyzes the conversions of 17-hydroxyprogesterone to 11-deoxycortisol and of progesterone to deoxycorticosterone. Therefore, deficiency in this enzyme results in increased levels of 17-hydroxyprogesterone and androstenedione. Simultaneous measurement of cortisol and androstenedione along with 17-hydroxyprogesterone can provide improved discrimination between truly affected and unaffected individuals, especially in neonates under stress. The inclusion of additional steroid hormones, such as 11-deoxycortisol, 11-dehydrocorticosterone, and corticosterone in the profiling panel would also promote improved discrimination between CAH subtypes. Moreover, it would help diagnose 11-hydroxylase deficiency, including the differential diagnosis of 11 β-hydroxylase 1 versus 11 β-hydroxylase 2 deficiency. In patients with 11 β-hydroxylase 1 deficiency, 11-deoxycortisol and 11-deoxycorticosterone levels are elevated to typically 20-300 times the upper limit of the reference range, while 11-deoxycortisol might or might not be elevated in patients with 11 β-hydroxylase 2 deficiencies.
In recent years, specific LC-MS/MS methods to analyze steroids in different matrices have been developed, but only few laboratories have applied it to CAH screening. Since the reference intervals of adrenal steroids are method-specific, the lack of available clinical data for the Korean population has been an obstacle in the clinical application of this method. In this study, we determined seven steroid levels in the Korean population. Samples from 76 preterm and 78 full-term neonates, 89 children, and 100 adults were analyzed. The results were in agreement with previously published data [222324].
In conclusion, we developed and validated a rapid and highly specific LC-MS/MS assay that quantifies seven endogenous steroids for CAH screening. With the expansion of newborn screening programs, a more specific method is needed for the diagnosis and follow-up of patients with CAH. Our LC-MS/MS-based method can circumvent common issues associated with immunoassays such as high false-positive rates and the inability to analyze multiple analytes in a single test. We propose that together with the validated method, the age-specific reference intervals in the Korean population will also help facilitate the clinical implementation of the method and improve the clinical assessment of patients.
Acknowledgments
This study was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A120030).
References
1. Rauh M. Steroid measurement with LC-MS/MS in pediatric endocrinology. Mol Cell Endocrinol. 2009; 301:272–281. PMID: 19007847.

2. Forest MG. Recent advances in the diagnosis and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Reprod Update. 2004; 10:469–485. PMID: 15514016.

3. Janzen N, Peter M, Sander S, Steuerwald U, Terhardt M, Holtkamp U, et al. Newborn screening for congenital adrenal hyperplasia: additional steroid profile using liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2007; 92:2581–2589. PMID: 17456574.

4. Riepe FG, Sippell WG. Recent advances in diagnosis, treatment, and outcome of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Rev Endocr Metab Disord. 2007; 8:349–363. PMID: 17885806.

5. Therrell BL. Newborn screening for congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2001; 30:15–30. PMID: 11344933.

6. Soldin SJ, Soldin OP. Steroid hormone analysis by tandem mass spectrometry. Clin Chem. 2009; 55:1061–1066. PMID: 19325015.

7. Stanczyk FZ, Clarke NJ. Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol. 2010; 121:491–495. PMID: 20470886.

8. Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA. Adrenal steroidogenesis in very low birth weight preterm infants. J Clin Endocrinol Metab. 1994; 78:266–270. PMID: 8106610.

9. Nordenström A, Wedell A, Hagenfeldt L, Marcus C, Larsson A. Neonatal screening for congenital adrenal hyperplasia: 17-hydroxyprogesterone levels and CYP21 genotypes in preterm infants. Pediatrics. 2001; 108:E68. PMID: 11581476.
10. Kim IS, Lee SY, Kim JW. An analysis of two-year experience in neonatal screening. Korean J Lab Med. 2004; 24:290–296.
11. Seo JY, Park HD, Kim JW, Oh HJ, Yang JS, Chang YS, et al. Steroid profiling for congenital adrenal hyperplasia by tandem mass spectrometry as a second-tier test reduces follow-up burdens in a tertiary care hospital: a retrospective and prospective evaluation. J Perinat Med. 2014; 42:121–127. PMID: 23989111.

12. Xu RN, Fan L, Rieser MJ, El-Shourbagy TA. Recent advances in high-throughput quantitative bioanalysis by LC-MS/MS. J Pharm Biomed Anal. 2007; 44:342–355. PMID: 17360141.

13. Holst JP, Soldin SJ, Tractenberg RE, Guo T, Kundra P, Verbalis JG, et al. Use of steroid profiles in determining the cause of adrenal insufficiency. Steroids. 2007; 72:71–84. PMID: 17157339.

14. Ministry of Health and Welfare and Planned Population Federation of Korea. Analysis of blood sample records for neonatal screening test and external quality assessment for inborn errors of metabolism in Korea. Seoul: Ministry of Health and Welfare;2013.
15. Lee DH. Newborn screening of inherited metabolic disease in Korea. Korean J Pediatr. 2006; 49:1125–1139.

16. Lacey JM, Minutti CZ, Magera MJ, Tauscher AL, Casetta B, McCann M, et al. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clin Chem. 2004; 50:621–625. PMID: 14656905.

17. Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003; 75:3019–3030. PMID: 12964746.

18. Horowitz GL, editor. Clinical and Laboratory Standards Institute. Defining, establishing, and verifying reference intervals in the clinical laboratory : approved guideline. Wayne, Pa.: Clinical and Laboratory Standards Institute;2008.
19. Kwon C, Farrell PM. The magnitude and challenge of false-positive newborn screening test results. Arch Pediatr Adolesc Med. 2000; 154:714–718. PMID: 10891024.

20. Rauh M. Steroid measurement with LC-MS/MS. Application examples in pediatrics. J Steroid Biochem Mol Biol. 2010; 121:520–527. PMID: 20036331.

21. Rossi C, Calton L, Hammond G, Brown HA, Wallace AM, Sacchetta P, et al. Serum steroid profiling for congenital adrenal hyperplasia using liquid chromatography-tandem mass spectrometry. Clin Chim Acta. 2010; 411:222–228. PMID: 19931522.

22. Mayo medical laboratories reference laboratory services for health care organizations. Accessed on Aug 2014. Available from http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/91196.
23. Janzen N, Sander S, Terhardt M, Peter M, Sander J. Fast and direct quantification of adrenal steroids by tandem mass spectrometry in serum and dried blood spots. J Chromatogr B Analyt Technol Biomed Life Sci. 2008; 861:117–122.

24. Kyriakopoulou L, Yazdanpanah M, Colantonio DA, Chan MK, Daly CH, Adeli K. A sensitive and rapid mass spectrometric method for the simultaneous measurement of eight steroid hormones and CALIPER pediatric reference intervals. Clin Biochem. 2013; 46:642–651. PMID: 23337690.

Fig. 1
Multiple reaction monitoring (MRM) transition of the respective steroids; intensity vs. time (min). The black peaks represent the target steroids. White peaks are steroids that have the same molecular weight with the target steroids.
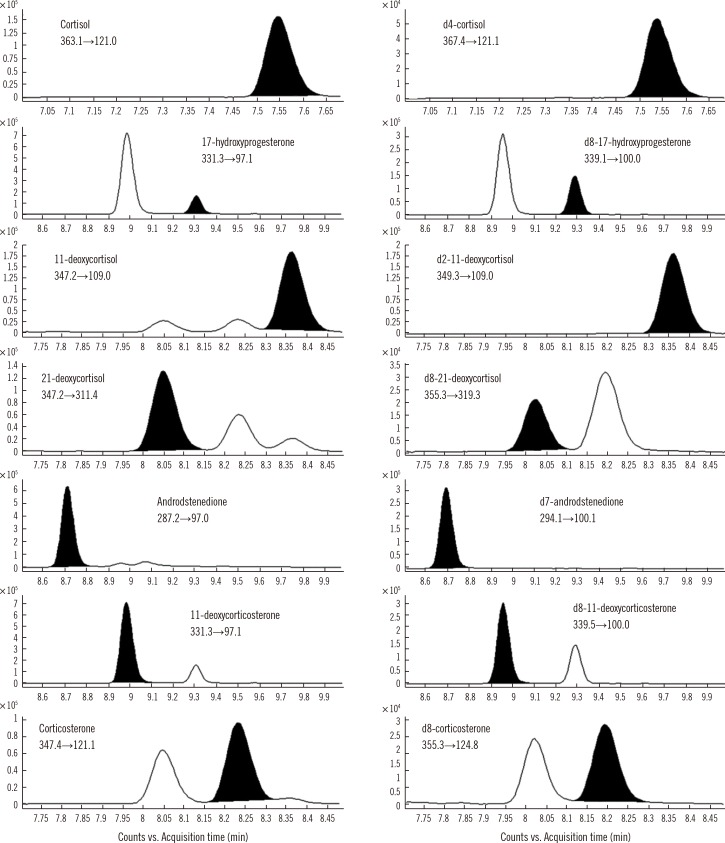
Table 1
HPLC-MS/MS operating conditions
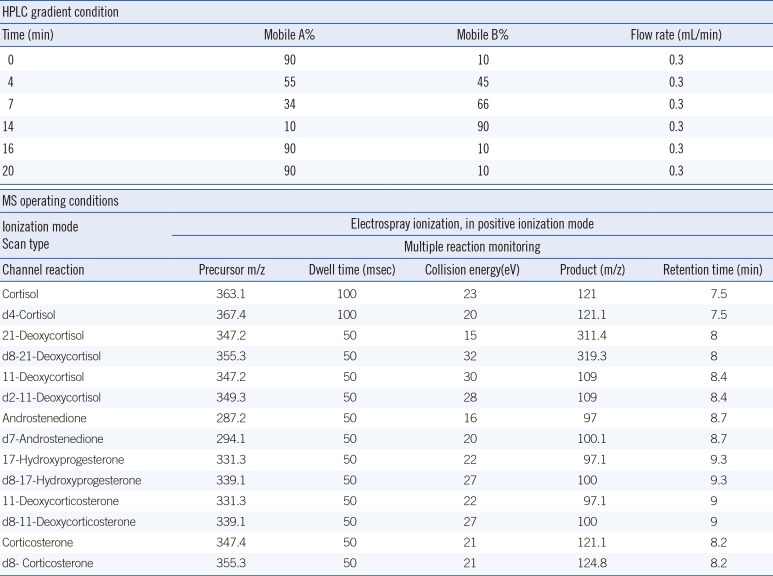
| HPLC gradient condition | |||||
|---|---|---|---|---|---|
| Time (min) | Mobile A% | Mobile B% | Flow rate (mL/min) | ||
| 0 | 90 | 10 | 0.3 | ||
| 4 | 55 | 45 | 0.3 | ||
| 7 | 34 | 66 | 0.3 | ||
| 14 | 10 | 90 | 0.3 | ||
| 16 | 90 | 10 | 0.3 | ||
| 20 | 90 | 10 | 0.3 | ||
Table 2
Precision, accuracy, linearity, and extraction recovery for the studied analytes
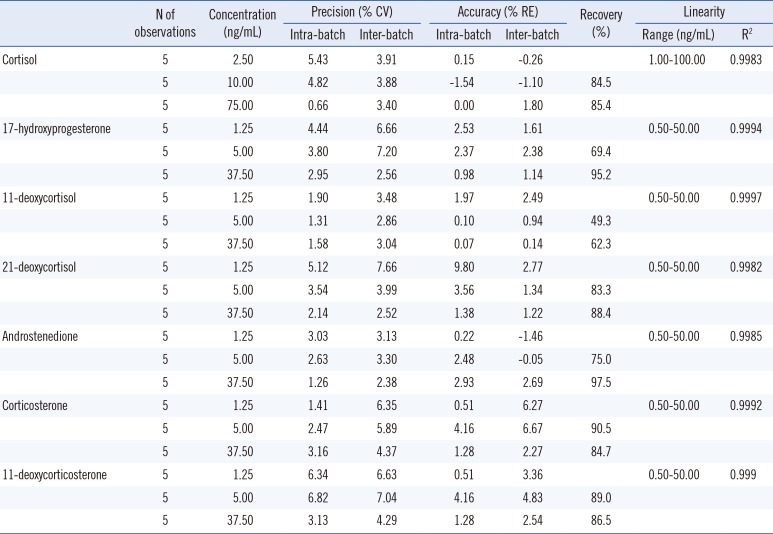
Table 3
Reference intervals of seven steroids in Korean subjects in comparison with previously published LC-MS/MS data




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download