Abstract
Background
Breast cancer is the most common type of cancer in females. Aberrant expression of microRNA-21 (miR-21) has previously been reported in breast cancer tissue. The aim of this study was to investigate expression levels of serum miR-21 in breast cancer patients and evaluate its prognostic value in Chinese females.
Methods
Real-time quantitative (RQ)-PCR was used to analyze miR-21 expression in archived serum, tumor tissue, and adjacent normal tissue from 549 participants (326 with breast cancer, 223 without breast cancer). We also analyzed associations between serum miR-21 expression and breast cancer subtypes and patient prognosis. Recurrence and survival were analyzed by using the multivariate Cox proportional hazards model.
Results
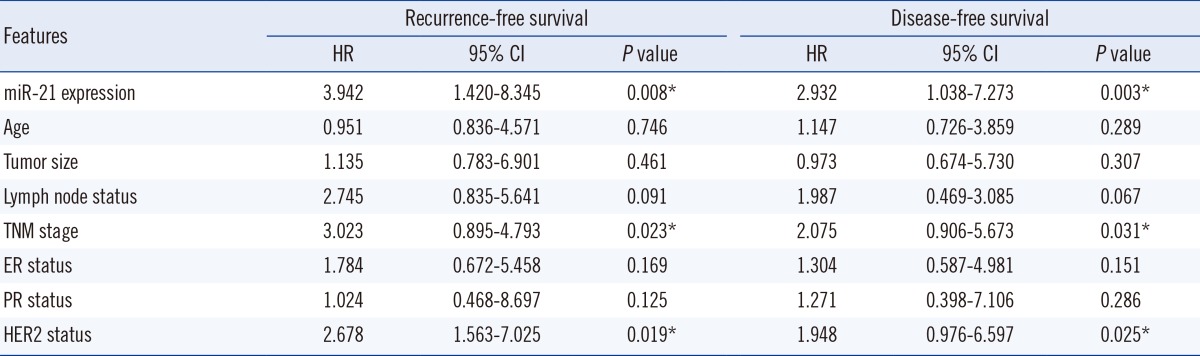
Expression of miR-21 was significantly higher in breast cancer tissues compared with normal adjacent breast tissues (P<0.001). The 2-ΔΔCt values for serum miR-21 in breast cancer patients versus healthy controls were 9.12±3.43 and 2.96±0.73, respectively. Multivariate Cox proportional hazards model suggested that serum miR-21 expression was an independent poor prognostic factor for both recurrence (hazard ratio [HR]= 2.942; 95% confidence interval [CI]=1.420-8.325; P=0.008) and disease-free survival (HR=2.732; 95% CI=1.038-7.273, P=0.003) in breast cancer.
In females, breast cancer (BC) is the most common type of cancer and the second leading cause of death from cancer [1]. Though recent studies have focused on elucidating the molecular mechanisms of BC, the exact mechanisms of BC initiation and progression remain unclear. Currently, conventional clinicopathological factors are insufficient for accurate prediction of BC patient outcomes. Therefore, there is a need for development of novel markers that predict the course of BC and that can potentially be used to develop treatment strategies.
MicroRNAs (miRNAs) are small (18-25 nucleotides long) non-coding RNA molecules that modulate the activity of specific messenger RNA (mRNA) targets involved in fundamental biological processes including development, apoptosis, proliferation, and differentiation. Following the discovery of lin-4 in Caenorhabditis elegans [2], thousands of miRNAs have been identified in a variety of organisms. Many studies have demonstrated that several miRNAs correlate with clinical and biological features of tumors, including tissue type, and are aberrantly expressed in various types of cancer [3, 4]. Many recent studies have identified expression of microRNA-21 (miR-21) in the serum of BC patients. Quantitative measurement of miR-21 expression has been previously reported in BC tissue [5]. These findings suggest that tumor-specific miRNAs in serum are derived not only from circulating blood cells but also from cancer cells. The miR-21 is a novel and promising biomarker for detection and diagnosis of biliary tract cancer [6, 7]. In particular, miR-21 has emerged as an oncogenic miRNA in BC, as it is the most consistently up-regulated miRNA in a wide range of cancers [8].
Here, we report that miR-21 plays a role in BC progression and has a prognostic value in a Chinese population. We investigated neoadjuvant chemotherapy treatment with either trastuzumab or lapatinib in BC patients based on molecular factors such as estrogen receptor (ER) status, human epidermal growth factor receptor 2 (HER2) positivity, or triple negative status. To address these issues, our study quantitatively measured serum miR-21 expression and determined its relationship to BC prognosis, including recurrence and survival, after resection.
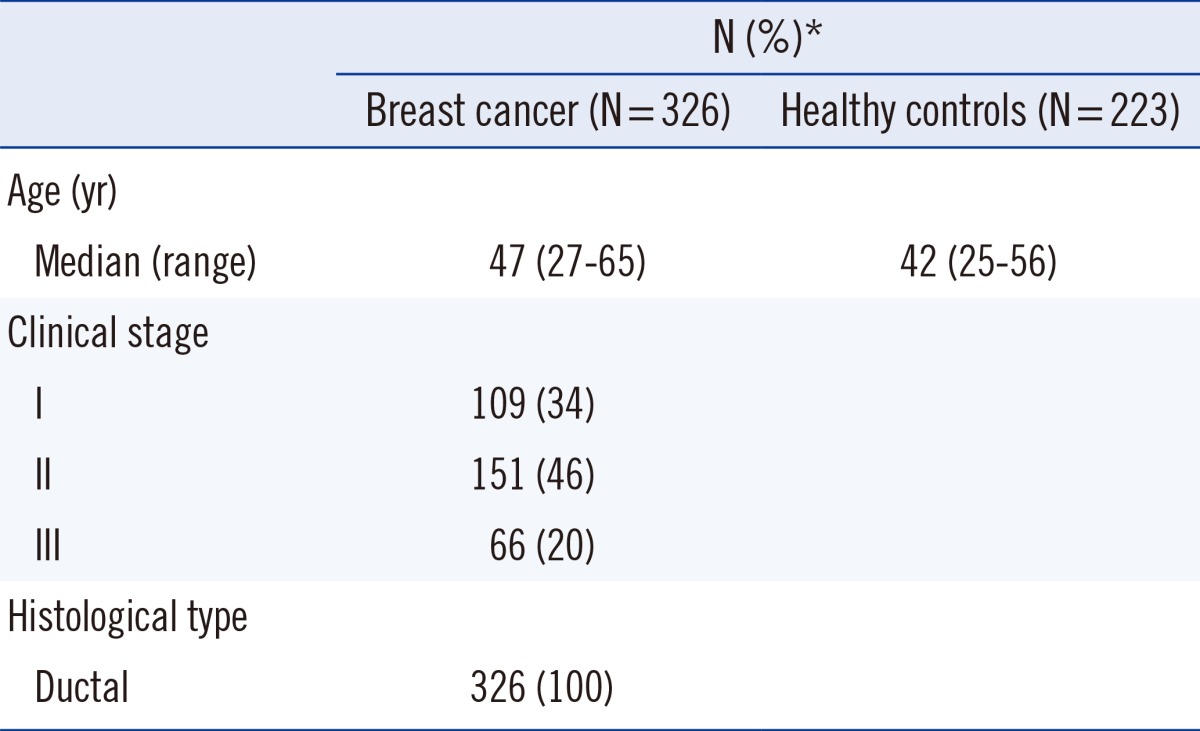
We examined 326 Chinese patients with invasive ductal carcinoma of the breast who underwent surgery between July 2009 and May 2014 at the First Hospital of Zibo City, China. Tissue and serum samples were collected from these 326 BC patients (154 patients in the trastuzumab arm and 172 patients in the lapatinib arm). For the validation study, serum samples were collected from 223 healthy volunteers. Healthy volunteers were confirmed to be free of malignant disease for >2 yr. Matched BC tumors and adjacent normal tissues were obtained from the First Hospital of Zibo City. Immediately after resection, tissue samples were frozen in liquid nitrogen and stored at -80℃ until examined. Whole blood samples were collected in Serum Separator Tubes (Becton Dickinson, Franklin Lakes, NJ, USA) during routine sampling from 223 healthy volunteers and 326 BC patients prior to treatment. Serum was harvested by centrifugation at 1,710 g for 10 min at 20℃ after allowing blood to clot for 30 min at 35℃. Fresh serum samples were then aliquoted into eppendorf tubes and immediately frozen at -80℃ until total RNA was isolated.
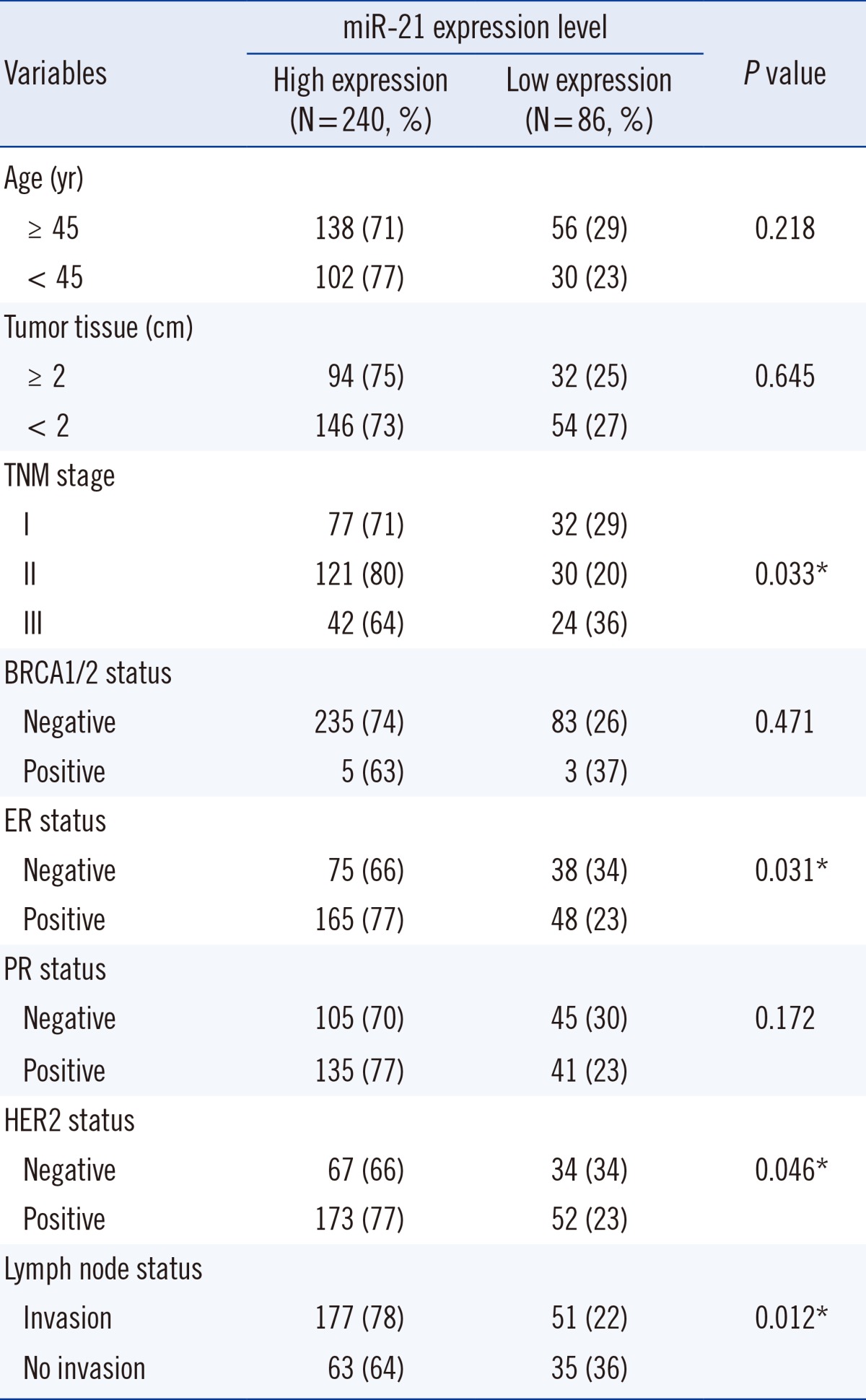
BC was staged according to 7th edition of the Cancer Staging Manual from the American Joint Committee on Cancer [9]. Recurrence was defined as a new tumor observed in the breast after initial curative resection. Progression was defined as disease with a higher tumor-node-metastasis stage at relapse. Diagnoses were independently confirmed as primary BC for all patients using histopathological records (BC 1/2, early onset [BRCA1/2] expression; ER/HER2 positivity; lymph node status; etc.). The Ethics Committee of the First Hospital of Zibo City approved this study, and written informed consent was obtained from all participants. For clinical results, miR-21 measurements were blinded. Detailed patient characteristics are listed in Table 1.
There were no differences in baseline characteristics between BC patients and normal subjects. Most of the patients with invasive ductal carcinoma of the breast had advanced-stage disease.
Total RNA was extracted from tumors, adjacent normal tissues, and serum samples using a commercial kit designed to isolate small molecular weight nucleic acids (miRNeasy Mini Kit; Qiagen, Venlo, Limburg, Netherlands). We performed RQ-PCR using the TaqMan miRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), which utilized stem-loop reverse primers. Reactions were carried out in an ABI Prism 7000 PCR system (Applied Biosystems). U6 small nuclear (sn) RNA was used as an endogenous control. The PCR conditions included initial incubation at 50℃ for 2 min and denaturing at 95℃ for 10 min, followed by 40 cycles of 95℃ for 15 sec and 60℃ for 60 sec [10]. All samples were measured in duplicate.
To estimate miR-21 expression, an expression index (EI) was calculated using the formula: 1,000×2(-ΔCt), where ΔCt is the difference in the threshold cycle (Ct) values between the target and the endogenous control used for analysis in the RQ-PCR results (ΔCt=CtmiR-21-CtU6 snRNA). The fold change in miR-21 expression for each BC sample was calculated relative to the average expression in normal control samples. Fold changes were calculated based on the Ct values using the following formula:
RQ=2-ΔΔCt, where ΔΔCt=(CtmiR-21-CtU6 snRNA)tumor-(CtmiR-21-CtU6 snRNA) mean normal.
All statistical analyses were performed by using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Values are presented as medians and ranges. Associations between the selected comparing groups were assessed by using Pearson's chi-square tests. Survival rates were calculated actuarially according to the Kaplan-Meier method, and survival curves were plotted; statistical differences were analyzed by using log-rank tests. The multivariate Cox proportional hazards model was used to assess the independent prognosis effect of serum miR-21 expression for risk of recurrence and disease-free survival. BC subtype and pathology factors included in the analysis were involved patient hormone positivity including HER2 positivity, triple negative status, and ER and progesterone receptor status. All tests comparing groups were 2-tailed, and P<0.05 was considered statistically significant.
We analyzed the expression levels of miR-21 in paired BC tissues and normal adjacent breast tissues from 326 BC patients. Analysis by RQ-PCR revealed that miR-21 expression was higher in BC tissues (median expression level=687.21; range= 98.95-4,981.31) than in normal adjacent breast tissues (median expression level=59.24; range=4.95-178.77; P<0.001).
Expression of miR-21 was detected in serum from BC patients, with expression levels that varied widely (EI values ranging from 25.4 to 9,230.2). The 2-ΔΔCt median expression of miR-21 was 0.67±0.15. We compared serum miR-21 expression between BC patients (n=326) and healthy controls (n=223). The 2-ΔΔCt values for BC patients versus healthy controls were 9.12±3.43 and 2.96±0.73, respectively. As shown in Fig. 1, association between serum miR-21 expression and tumor-node-metastasis stage were found to be up-regulated in BC patients (P=0.033).
We first performed survival analysis for all patients, and then for BC subtypes with differences in hormone positivity including ER status, HER2 positivity, or triple negative status (Table 2). The relationship between miR-21 expression and prognosis (recurrence or survival) varied by disease stage. We investigated the serum levels of miR-21 in 326 BC patients before neoadjuvant chemotherapy treatment along with clinicopathological characteristics and pathological complete responses to trastuzumab or lapatinib-based neoadjuvant therapy. Our subgroup analysis revealed that recurrence was significantly different between patients with high versus low miR-21 expression.
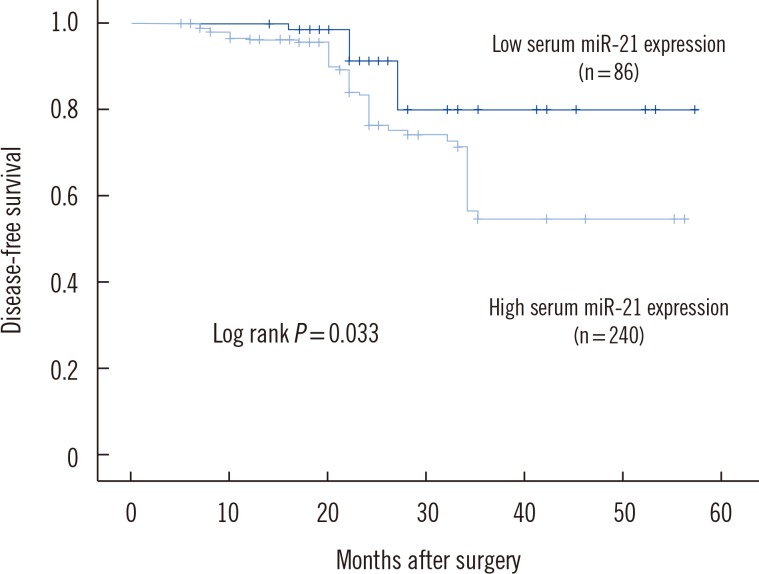
To determine whether elevated serum miR-21 was a potential predictor of prognosis in BC patients, recurrence-free and disease-free survival were analyzed, with the BC patients divided into subgroups according to serum miR-21 expression level. Kaplan-Meier analysis demonstrated differences in the recurrence-free and disease-free survival curves in patients with high versus low expression of serum miR-21. As shown in Fig. 2, the high serum miR-21 expression group had a significantly shorter recurrence-free survival than in the low serum miR-21 expression group (P=0.026). Moreover, disease-free survival was significantly shorter in the high serum miR-21 expression group than in the low serum miR-21 expression group (P=0.033) (Fig. 3).
The multivariate Cox proportional hazards model indicated that serum miR-21 expression was an independent poor prognostic factor for both recurrence (HR=2.942; 95% CI=1.420-8.325; P=0.008) and disease-free survival (HR=2.732; 95% CI=1.038-7.273; P=0.003) in HER2-positive BC patients receiving neoadjuvant therapy (Table 3).
In this study, we examined expression of miR-21 in tissues and serum from 326 BC patients and 223 healthy controls. We found that serum levels of miR-21 were significantly higher in BC patients than in controls. This is in contrast to a study by Mar-Aguilar et al. [11] that found similar levels of miR-21 expression in BC patients and controls. Similarly, when Chan et al. [12] reported the 20 miRNAs that were most significantly differentially expressed in BC tumors, they found that only seven miRNAs were overexpressed in both tumors and serum, and miR-21 was not identified as the most important diagnostic marker. Also, miR-21 levels in serum showed a number of small differences between BC patients who developed metastasis and BC patients who remain metastasis-free [12].
Notably, miR-21 is one of the most frequently studied oncogenic miRNAs (onco-miRNAs). Although a direct correlation between aberrant expression of miR-21 and BC has been previously demonstrated, it is not clear whether suppression of miR-21 alone will affect tumorigenesis [13]. In this report, we showed that miR-21expression was significantly higher in BC tissues than in normal adjacent breast tissues (Table 1).
The miR-21 gene is located on chromosome 17q23.2, which is inside the common fragile site FRA17B. This region is frequently amplified in breast and lung cancer [14]. In BC, miR-21 is an onco-miRNA. Elevated miR-21 expression may facilitate tumor progression, and miR-21 expression may be up-regulated by transforming growth factor-β [15]. This study provides a comprehensive investigation of the association between serum miR-21 and BC prognosis in Chinese females. We found an association between serum miR-21 expression and prognosis (recurrence and survival) that seemed to vary by disease stage. Patients with high serum miR-21 expression had an increased risk of disease progression compared to patients with low serum miR-21 expression (Table 2).
There is a large amount of evidence indicating that miR-21 is associated with regulation of proliferation and differentiation during development. Also, it is possible to develop treatment strategies by targeting miR-21 [16]. Serum miRNA levels show a number of small differences in females who later develop cancer versus those who remain cancer-free. Microarray analysis of miRNAs in the serum of BC patients has only recently been reported. Aberrantly expressed miR-21 in the blood of BC patients is occasionally reported, and expression of miR-21 and miR-146a in plasma samples from BC patients might be useful for BC diagnosis in Indian females [17]. Our results show that expression of serum miR-21 is up-regulated in BC patients compared to healthy controls in a population of Chinese females. High expression of serum miR-21 may contribute to BC pathogenesis (P=0.033) (Fig. 1) and development of invasion and lymph node metastasis (P=0.012) (Table 2).
Although the impact of miR-21 on BC prognosis remains controversial, a few studies have reported promising associations with miR-21 expression levels that could make this a novel potential biomarker for BC prognosis. Previously, Gao et al. [18] reported that serum miR-21 was a more sensitive BC marker than cancer antigen (CA)15-3 or carcinoembryonic antigen; in particular, miR-21 was a potential tumor marker for the diagnosis of early-stage BC. Also, miR-21 levels before and after chemotherapy demonstrated a detectable association with overall survival that was independent of anti-HER2 therapy. Investigation of serum miR-21 has yielded encouraging data that suggests that the clinical relevance of miR-21 should be confirmed in a larger BC patient cohort [19]. When BC is suspected, clinicians may check factors such as CA15-3, Ki-67, cytokeratin 8/18/19, BRCA1/2, and ER/HER2, which are still the main diagnostic biomarkers [20, 21]. The BRCA1 and BRCA2 tumor suppressor genes are strong predictors of BC development; mutations in BRCA 1 and 2 are inherited in an autosomal dominant manner and play important roles in BC risk [22]. Reports have indicated that miR-21 functions as an oncogene and modulates tumorigenesis through regulation of genes such as bcl-2; therefore, miR-21 may serve as a novel therapeutic target [23].
Though new technologies are emerging to improve the specificity, stability, and efficiency of miRNA delivery and therapy, the final outcomes of this strategy remain uncertain [24]. Currently, clinicians do not have an effective molecular biology marker for BC. Expression profiles of circulating miRNAs may yield promising biomarkers for diagnosis and assessment of the prognosis of cancer patients. Sensitive techniques allow the expression levels of many miRNAs to be determined, and this information can be used for diagnostic purposes [25]. The mechanism underlying miRNA-21 stability is still being investigated for BC detection [26].
The utility of miRNA profiles as potential diagnostic or prognostic markers for BC has been gaining interest [27]. Our study had some limitations, including small sample size and a limited ability to generalize our results since all our patients were Chinese females. Despite these limitations, our study provided initial data about the up-regulation of serum miR-21 in BC patients and suggested the diagnostic value of serum miR-21 in BC patients with metastasis. Serum miR-21 may have clinical utility for monitoring and follow-up of BC patients with metastasis. Clearly, our results should be further validated by a prospective study in a multicenter clinical trial. The ideas in this study should be further explored by studies with larger sample numbers.
In conclusion, we found that serum miR-21 was up-regulated in BC patients, and increased miR-21 expression levels correlated with poor prognosis of BC patients. Our findings indicate that serum miR-21 may serve as a novel potential prognostic marker for recurrence and survival of BC patients before resection.
Acknowledgments
This study was supported in part by the Research Program Foundation (No.2013) of the First Hospital of Zibo City, and the Natural Scientific Foundation of Shandong Province (grant No. ZR2010CQ031).
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90. PMID: 21296855.

2. Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993; 75:855–862. PMID: 8252622.

3. Michael MZ, O'Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003; 1:882–891. PMID: 14573789.
4. Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, yendamuri S, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004; 101:2999–3004. PMID: 14973191.

5. Wang F, Zheng ZG, Guo J, Ding X. Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol Oncol. 2010; 119:586–593. PMID: 20801493.

6. Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011; 8:467–477. PMID: 21647195.

7. Kishimoto T, Eguchi H, Nagano H, Kobayashi S, Akita H, Hama N, et al. Plasma miR-21 is a novel diagnostic biomarker for biliary tract cancer. Cancer Sci. 2013; 104:1626–1631. PMID: 24118467.

8. Zhao H, Shen J, Medico L, Wang D, Ambrosone CB, Liu S. A pilot study of circulating miRNAs as potential biomarkers of early stage breast cancer. PLos One. 2010; 5:e13735. PMID: 21060830.

9. Edge SB, Byrd DR, Carducci M, Compton CC, Fritz AG, Greene FL, editors. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag;2009.
10. Asaga S, Kuo C, Nguyen T, Terpenning M, Giuliano AE, Hoon DS. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011; 57:84–91. PMID: 21036945.

11. Mar-Aguilar F, Mendoza-Ramíreza JA, Malagón-Santiago I, Espino-Silva PK, Santuario-Facio SK, Ruiz-Flores P, et al. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis Markers. 2013; 34:163–169. PMID: 23334650.

12. Chan M, Liaw CS, Ji SM, Tan HH, Wong CY, Thike AA, et al. Identification of circulating microRNA signatures for breast cancer detection. Clin Cancer Res. 2013; 19:4477–4487. PMID: 23797906.

13. Iorio MV, Ferracin M, Liu CG, Veronesse A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005; 65:7065–7070. PMID: 16103053.

14. Calin GA, Croce CM. Chromosomal rearrangements and microRNAs: a new cancer link with clinical implications. J Clin Invest. 2007; 117:2059–2066. PMID: 17671640.

15. Qian B, Katsaros D, Lu L, Preti M, Durango A, Arisio R, et al. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-β1. Breast Cancer Res Treat. 2009; 117:131–140. PMID: 18932017.

16. Yan LX, Wu QN, Zhang Y, Li YY, Liao DZ, Hou JH, et al. Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast Cancer Res. 2011; 13:R2. PMID: 21219636.

17. Kumar S, Keerthana R, Pazhanimuthu A, Perumal P. Overexpression of circulating miRNA-21 and miRNA-146a in plasma samples of breast cancer patients. Indian J Biochem Biophys. 2013; 50:210–214. PMID: 23898484.
18. Gao J, Zhang Q, Xu J, Guo L, Li X. Clinical significance of serum miR-21 in breast cancer compared with CA153 and CEA. Chin J Cancer Res. 2013; 25:743–748. PMID: 24385703.
19. Müller V, Gade S, Steinbach B, Loibl S, von Minckwitz G, Untch M, et al. Changes in serum levels of miR-21, miR-210, and miR-373 in HER2-positive breast cancer patients undergoing neoadjuvant therapy: a translational research project within the Geparquinto trial. Breast Cancer Res Treat. 2014; 147:61–68. PMID: 25086636.

20. Tamaki K, Ishida T, Tamaki N, Kamada Y, Uehara K, Miyashita M, et al. Analysis of clinically relevant values of Ki-67 labeling index in Japanese breast cancer patients. Breast Cancer. 2014; 21:325–333. PMID: 22782361.

21. Leivonen SK, Mäkelä R, Ostling P, Kohonen P, Haapa-Paananen S, Kleivi K, et al. Protein lysate microarry analysis to identify microRNAs regulating estrogen receptor signaling in braest cancer cell lines. Oncogene. 2009; 28:3926–3936. PMID: 19684618.
22. Fackenthal JD, Olopade Ol. Breast cancer risk associated with BRCA1and BRCA2 in diverse populations. Nat Rev Cancer. 2007; 7:937–948. PMID: 18034184.
23. Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007; 26:2799–2803. PMID: 17072344.

24. Li J, Shen L, Xiao XG, Fang L. MicroRNAs in breast cancer and breast cancer stem cells and their potential for breast cancer therapy. Chin Med J (Engl). 2013; 126:2556–2563. PMID: 23823834.
25. Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010; 102:1174–1179. PMID: 20234369.

26. Wu Q, Wang C, Lu Z, Guo L, Ge Q. Analysis of serum genome-wide microRNAs for breast cancer detection. Clin Chim Acta. 2012; 413:1058–1065. PMID: 22387599.

27. Fu SW, Chen L, Man YG. miRNA biomarkers in breast cancer detection and management. J Cancer. 2011; 2:116–122. PMID: 21479130.

Fig. 1
Association between serum microRNA-21 (miR-21) expression and tumor-node-metastasis (TNM) stage. Significant: 2-sided Pearson's chi-square test, P<0.05.
Abbreviations: miR-21, microRNA-21; TNM, tumor-node-metastasis.
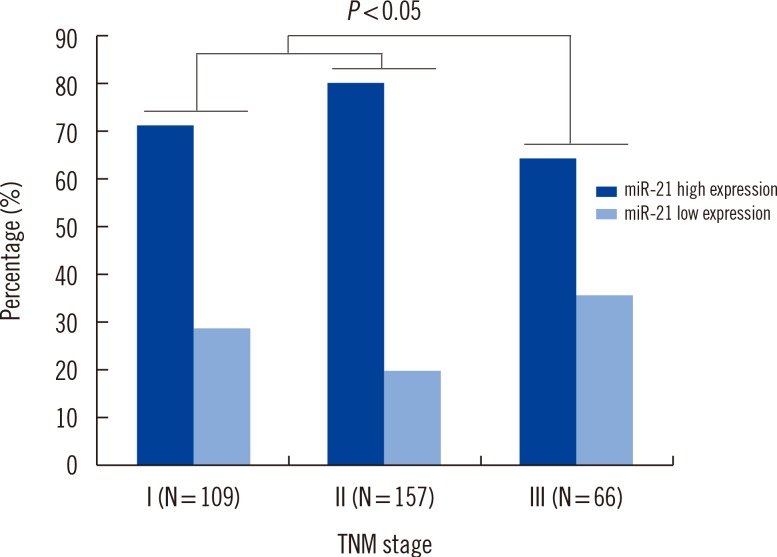
Fig. 2
Kaplan-Meier curves for recurrence-free survival in 326 breast cancer patients. Recurrence-free survival was significantly lower in patients with high serum miR-21 expression than in patients with low serum miR-21 expression.
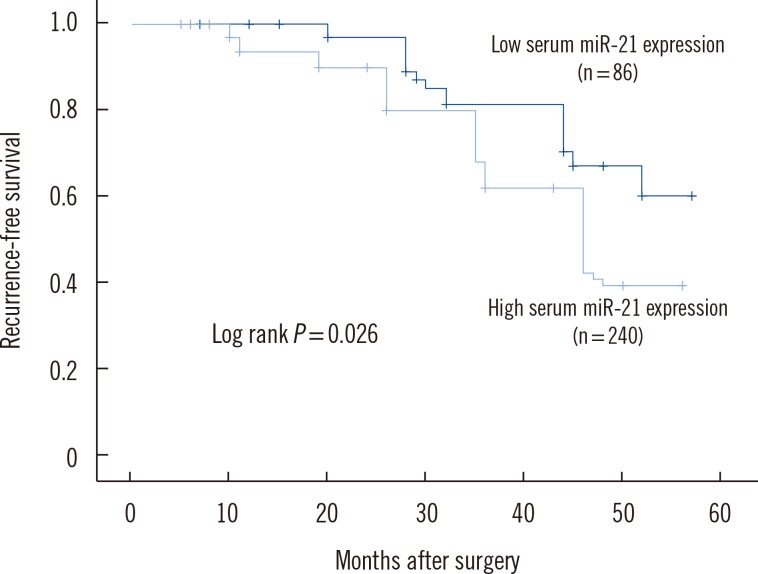
Fig. 3
Kaplan-Meier curves for disease-free survival in 326 breast cancer patients. Disease-free survival was significantly lower in patients with high serum miR-21 expression than in patients with low serum miR-21 expression.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download