Abstract
After the relationship between glycemic control and the HbA1c concentration was demonstrated, many tests have been developed to determine the HbA1c concentration. The test results are standardized to the International Federation of Clinical Chemistry (IFCC) Reference Measurement Procedure (RMP) in harmony with the efforts of the National Glycohemoglobin Standardization Program (NGSP). The longitudinal use of the test requires strict quality management including accreditation of the laboratory, a dedicated internal control design, participation in an external quality assessment (EQA) program (proficiency test), and careful consideration of pre- and post-analytical aspects of the test. Performance goals for optimizing determination of the HbA1c concentration have been described. As an index of long-term glycemic control and a risk predictor, the HbA1c concentration is an indispensable part of routine management of diabetes. Because of the improving quality of the test, the HbA1c concentration is being increasingly applied in the diagnosis of diabetes. There are, however, concerns of this application in point-of-care settings. The HbA1c concentration is also used to achieve stringent control in pregnant diabetic patients. Strict standardization enables the definition of universal reference values and clinical decision limits. This review describes the present status of analytical and clinical aspects of determining the HbA1c concentration and highlights the challenges involved.
Diabetes is characterized by chronic hyperglycemia and causes long-term complications like retinopathy, neuropathy, and nephropathy. It generally accelerates macro- and micro vascular changes. Because of lifestyle changes (i.e., eating more and exercising less), diabetes has become a global epidemic. Approximately 346 million people have been diagnosed with diabetes worldwide, and in the US, diabetes-related conditions account for 1 of every 7 dollars in the health care budget [1, 2]. Efficient and effective management is required to handle this epidemic. Portable glucose meters facilitate the short-term management of diabetes. Long-term prospective studies, notably the Diabetes Control and Complications Trial (DCCT), the UK prospective Diabetes Study Group (UKPDS), and the Epidemiology of Diabetes Interventions and Complications (EDIC) study, have provided definite evidence that diabetic complications are directly related to mean glycemia value, as measured by the HbA1c concentration [3-5]. This, along with successful standardization, has made the HbA1c concentration a valuable diagnostic tool for monitoring long-term glycemic control as well as defining specific treatment targets and decision limits. We review and discuss the analytical and clinical aspects as well as the remaining challenges of detecting the level of HbA1c.
The relevance of the HbA1c concentration in diabetes management is well recognized by the diagnostic industry, and various commercial tests have been developed in this respect [6].
There are two major analytical concepts: one, based on the separation of Hb fractions and the other, based on chemical reactions (Fig. 1). Although different analytes are measured by these methods, assays can be standardized according to the Reference Measurement Procedure (RMP) of the International Federation of Clinical Chemistry (IFCC) [7, 8].
The fact that HbA1c and non-glycated Hb have different chemical properties allows for the separation of fractions and the quantification of HbA1c. This principle is applied in ion exchange chromatography (IEC), capillary electrophoresis (CE), and affinity chromatography (AC).
IEC: The pI of HbA1c and Hb differ by 0.02 units. This difference is sufficient to allow for the separation of HbA1c from non-glycated Hb via HPLC. With IEC, fetal Hb (HbF), minor fast Hb (HbA1a/b), and carbamylated Hb (HbCarb) as well as genetic variants (e.g., sickle cell Hb) can also be visualized [6].
CE: This method uses the charge difference between HbA1c and other Hb fractions. Separation is achieved via a high-voltage electrical field and electroosmotic flow [9].
AC: While non-glycated Hb runs freely through a column packed with boronic acid coated particles, glycated Hb molecules have an affinity to boronic acid, and HbA1c is retained on the column. This is the basis for separation. In addition to the N-terminal valine of the β-chain (HbA1c), glucose binds to approximately 15 other lysine residues in Hb. Altogether, these other glycated Hb account for approximately a half of all detected HbA1c molecules. As they are formed proportionally to HbA1c, calibration enables the results of the AC test to be expressed in terms of HbA1c [10].
In chemical tests, HbA1c concentration is measured based on a specific chemical reaction to the glycated N-terminal valine of the β-chain. Total Hb concentration is measured in parallel with photometry. Two independent tests, HbA1c and total Hb tests, are therefore required to calculate the HbA1c concentration. This concept is applied in immunochemical and enzymatic assays.
Immunoassays (IA): In IA, an excess of anti-HbA1c antibodies is added to a hemolyzed sample. After binding to HbA1c, the excess antibodies agglutinate. The turbidity of the resulting immunocomplexes is measured photometrically using a turbidimeter or nephelometer. In parallel, the total Hb concentration is measured bichromatically in the pre-incubation phase [11].
Enzymatic assays: A protease cleaves the β-chain to liberate peptides. Peptides, usually dipeptides, react with fructosyl peptide oxidase, and the resulting hydrogen peroxide is used to quantify HbA1c. In parallel, the total Hb concentration is measured photometrically [12].
The HbA1c concentration is a longitudinal parameter; patients are monitored over years or even decades. Therefore, a reliable test with highly reproducible results over a long period is required. For convenience, HbA1c measurements should preferably be available during the patient's visit to the physician or even be performed in front of the patient. Determining the HbA1c concentration is a high-volume test and therefore demands efficiency, a high throughput, robustness, and cost efficiency. The chosen method should also match the organizational structure i.e., it should be integrated into the general chemistry analyzer, a convenient stand-alone laboratory instrument, or as a point-of-care (POC) instrument in the doctor's office [6]. Priorities will differ according to the situation. Thus, the weight given to the strengths and weaknesses should be considered.
IEC meets the requirements of high throughput, quality, and robustness. Hb variants are seen, which can be regarded as a strength (detection of carriers and genetic counseling) or weakness (complications in routine laboratories and interference of HbA1c). A dedicated stand-alone instrument can also be a weakness. The same characteristics generally apply for CE. The runtime is considerably longer, which leads to better separation (no complications with variants). However, parallel capillaries must be operated to achieve high throughput. It can be challenging to achieve consistent results with all capillaries. If a robust column is required, AC can afford the same analytical quality as IEC, and variants are not seen. Chemical methods have the advantage that tests can simply be performed using general chemistry analyzers. However, the two independent tests required may negatively impact the analytical quality. Variants are not detected and do not interfere in the measurement. The analytical principles described apply to both laboratory and POC instruments. The strength of POC is its convenience of use, i.e., it requires no additional visit to the laboratory. A weakness is the questionable quality of the test that stems from the performance of the test itself or improper handling by non-laboratory staff.
More than 100 commercial tests have been developed based on the five analytical principles described above. New assays, as well as modified versions of existing ones, continuously enter the market. It would be impossible to address all of them, and many are now obsolete. This review discusses only the general strengths and weaknesses. Up-to-date information on tests can be obtained from external quality assessment (EQA) or proficiency test (PT) programs.
The most common interferences are Hb variants, elevated levels of HbF, and derivatives. Structural Hb variants have point mutations in the protein chains. Nine hundred variants have been identified, but 99% fall into four categories: S (high prevalence in black Africans and Americans, those of Mediterranean decent, and Indians), C (black Africans and Americans), E (South East Asian), and D (more equally spread over the globe). Synthetic variants arise from genetically determined changes in the capacity to synthesize Hb chains. In β-thalassemia, the production of β-chains is inhibited. When Hb molecules are assembled, β-chains are replaced by γ- or δ-chains, thereby elevating the levels of HbF and HbA2, which are normally very low. Derivatives (also called adducts) result from the posttranslational modification of Hb. Apart from HbA1c, the most common derivatives are HbCarb and pre-HbA1c (a Schiff base) [13].
The heterozygous forms of S, C, D, and E do not cause hemolytic diseases. As they all have a terminal β-valine, glycation is the same as that for HbA1c. The analytical interference of these Hbs is variant- and method-specific and is difficult to generalize [14]. The NGSP systematically evaluated most methods, and a periodically updated review can be found on the website [15]. As HbF has no β-chain and the γ-chain has a terminal glycine instead of a valine, HbF can only be glycated at lysine residues, resulting in a glycation rate of approximately one-third of that of HbA. With IA, the HbA1c concentration will decrease by 1%, and with AC, it will decrease by 0.7% for every 1% HbF. This interference becomes substantial at levels exceeding 10-15%. Both IEC and CE can generally separate HbF from HbA1c. The pI of HbCarb and pre-HbA1c roughly equals that of HbA1c. In older IEC methods, both HbCarb and pre-HbA1c would co-elute with HbA1c. In most instruments currently available in the market, they are separated well enough to prevent interference.
Different specificities and selectivities of routine methods lead to broad variation. However, optimal clinical use requires equivalence of results, regardless of the method used. Only then can the universal guidelines with uniform reference values and decision limits (and thus easy interpretation and comparability of scientific studies) be adhered to. Equivalence can be achieved through harmonization (calibration of routine methods against an arbitrarily designated comparison method) or standardization (calibration against a reference measurement procedure of higher metrological order) [16].
The well-recognized need for equivalence initiated national harmonization efforts. In the US, the arbitrarily chosen method was the method used as the anchor in the DCCT Study. The nationwide program with international affiliations was, and still is, organized by the NGSP [17]. Similar programs resulted in harmonization in Japan (Japan Diabetes Society/Japanese Society for Clinical Chemistry) and Sweden (Mono-S) [18, 19]. Unfortunately, the methods of these harmonization efforts yielded variable results.
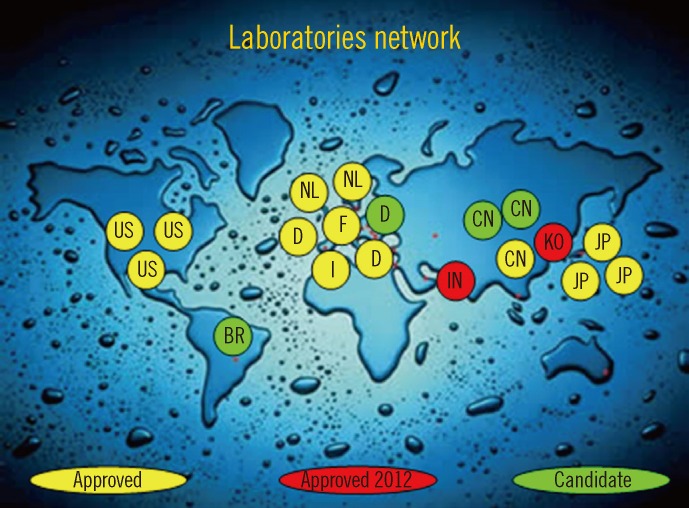
The aforementioned situation created confusion. The IFCC therefore developed a reference measurement procedure of higher metrological order (IFCC-RMP) to achieve worldwide standardization [7, 19]. The IFCC-RMP is based on the concept of metrological traceability. Pure HbA1c and HbA0 are mixed to calibrate the IFCC-RMP. Hb-containing washed and lysed erythrocytes are cleaved with endoproteinase, and the resulting hexapeptides are measured with either HPLC-CE or HPLC-electrospray mass spectrometry. With the IFCC-RMP, values are assigned to whole blood panels that serve as calibrators for manufacturers. In this way, the complete quality chain from IFCC-RMP to patient is created (Fig. 2). The IFCC-RMP is embedded in a global network of reference laboratories in Europe, Asia, and the US [20] (Fig. 3).
The ultimate aim of the IFCC is the global standardization of all routine methods. The HbA1c concentration of patients should be reported in the International System (SI) of units. Unfortunately, universal reporting will unlikely be achieved; there was, and still is, resistance against a change of units [21]. To solve this dilemma, the IFCC and the major diabetes organizations-the International Diabetes Federation (IDF), the European Association for the Study of Diabetes (EASD), and the American Diabetes Association (ADA)-agreed on a consensus statement [22]. The major statements are: 1) the IFCC-RMP for HbA1c represents the only valid anchor to implement standardization of the measurement; 2) HbA1c results are to be reported by clinical laboratories worldwide in SI units (i.e., mmol/mol) and derived NGSP/DCCT units (%); and 3) editors are strongly recommended to report HbA1c concentrations in both units. However, reporting patient results in both units is unpractical, and the HbA1c concentration is often reported in only one set of units.
The master equations for converting IFCC units into NGSP units (NGSP%=0.0915×IFCC mmol/mol+2.15) and vice versa (IFCC mmol/mol=10.93 NGSP%-23.5) are established and monitored by the IFCC and NGSP networks [20].
In summary, full (all routine methods are calibrated against the IFCC-RMP) and partial standardization of reported patient results (IFCC and NGSP units) have been established.
The HbA1c concentration is the ultimate longitudinal parameter; therapy depends on serial HbA1c measurements over a period of years or decades. Therefore, considerable attention should be given to quality management.
Unlike glucose samples, HbA1c samples can be easily collected and stored. Blood can be taken at any time without patient precautions. Blood obtained by venipuncture or finger-stick capillary is suitable. Unless otherwise specified by the manufacturer, the anticoagulant should be EDTA. Sample stability is method specific. HPLC methods are most sensitive to aging effects. Some POC tests cannot measure even slightly hemolyzed specimens. Blood is generally stable for up to 1 week at 2-8℃. Blood stored below -70℃ is stable for at least 1 yr. Storage at -20℃ has adverse effects and should be avoided [23]. Some manufacturers have developed method-specific collection systems to facilitate field-collection (e.g., filter paper and micro-cups with lysing buffer). These systems should only be used if validated in comparison with standard collection [21].
Results below the lower limit of the reference interval should be confirmed with repeated testing. If confirmed, the clinician should be informed about the possibility of variant or shortened erythrocyte survival. Samples with extremely high results (>140 mmol/mol or >15%) and those for which the results do not match the clinical picture should be re-assayed. Repeated testing with a different analytical method can help detect the reason for unexpected patient- or method-related results.
In qualified laboratories, a quality system consisting of three basic principles is in place: accreditation to the International Organization for Standardization (ISO) 15189, internal QC, and EQA. For internal control, two control samples (with low and high HbA1c concentrations) should be assayed in every analytical run. Frozen whole-blood aliquots stored below -70℃ and lyophilized hemolysates with no or known matrix effects are suitable [21]. Participation in an EQA or PT program provides valuable external information for managing the quality of the HbA1c test. Bias is derived from the EQA target set with the IFCC-RMP, performance is compared with other laboratories using the same method, and EQA reports provide an up-to-date review of available methods and their performances.
The reliability of HbA1c measurement depends on bias (related to proper calibration) and precision (related to the reproducibility of the method). Quality goals can be derived from biological variation, clinical needs, or the state of the art. For HbA1c, a generally accepted rule of thumb is that clinicians interpret a difference of 5 mmol/mol (0.5%) between successive patient samples as a significant change in glycemic control [24]. Therefore, the intralaboratory CV (derived from the lab's internal QC records) should be <3% (<2% NGSP units). The overall interlaboratory CV (derived from the EQA review) should be <5% (<3.5% NGSP units) or <4.5% (<3% NGSP units) within one method [21]. The difference in performance goals is derived from the unspecificity of the NGSP reference method [25].
The quality concept used in laboratories is not usually applied to POC testing settings. Careful reading of the instructions, checking if the manufacturer warrants traceability of results to the IFCC-RMP, and periodic exchange of samples with a qualified laboratory, all contribute to the quality of the test. Participation in an EQA program is strongly advised. In situations where no EQA is available, a professional organization or the government should carry out EQA.
The HbA1c concentration is widely used for the routine monitoring of long-term glycemic status in both type I and type II diabetes patients. HbA1c is the index of mean glycemia and as such, documents the degree of glycemic control, response to therapy, and risk for developing or worsening diabetes complications [3, 26].
Considering improved standardization of the test and recent data demonstrating the relation with retinopathy, an international expert committee recommended using the HbA1c concentration for diagnosing diabetes. This view has been adopted in many countries including the US, Japan, and the United Kingdom. However, application in daily practice varies from replacing the glucose tolerance test and/or fasting plasma glucose to using HbA1c measurements in parallel to these tests [27-29]. The WHO recommends that "HbA1c can be used as a diagnostic test for diabetes providing that stringent quality assurance tests are in place and assays are standardized to criteria aligned to the international reference values, and there are no conditions present that preclude its accurate measurement." These conditions include pregnancy, suspected type I diabetes, a short duration of diabetes symptoms, acute illnesses, receiving medication that may cause a rapid increase in the glucose level, pancreatic damage, hemoglobinopathies, anemia, renal failure, and HIV infection [30].
A specific application is the use of HbA1c measurements during pregnancy in patients with diabetes to determine the minimal perinatal risk for the mother and maximum health of the fetus. Stringent control prior to and during pregnancy decreases the risk of congenital malformations, overweight infants, as well as complications of pregnancy and delivery related to poorly managed glycemic control [31].
Health care authorities indirectly use HbA1c measurements when assessing the quality of diabetes care. The (frequency of) use by health care providers and the mean value or proportion of patients below a specific target is monitored [32].
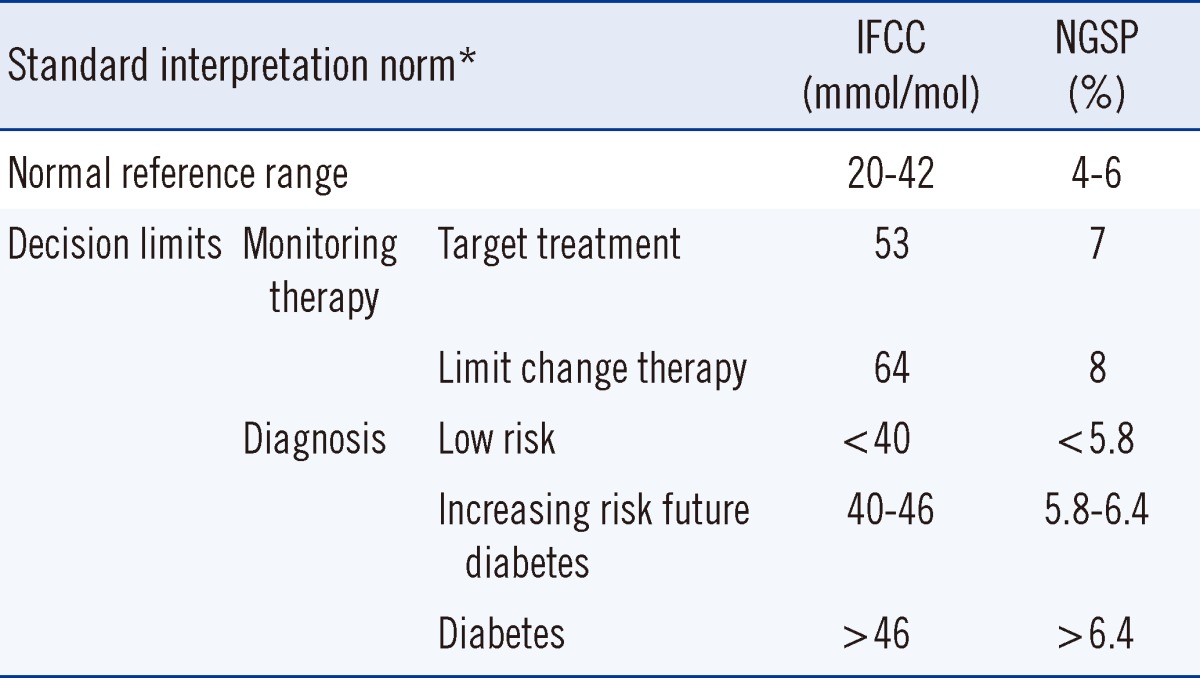
The normal reference range and clinical decision limits for IFCC/NGSP-standardized HbA1c concentration are summarized in Table 1. The normal reference range is derived from the landmark DCCT Study [3]. The general target for treatment of diabetes patients is 53 mmol/mol (7%) with the recommendation to strengthen therapy when the level of HbA1c exceeds 64 mmol/mol (8%) [26]. The HbA1c concentration represents a continuum: values <40 mmol/mol (5.8%) indicate a low risk for diabetes, whereas those >46 mmol/mol (6.4%) indicate the presence of diabetes. HbA1c concentrations of 40-46 mmol/mol (5.8-6.4%) are associated with an increasing risk of diabetes [26]. Serial measurements may improve the HbA1c concentrations in type I patients, but there is no consensus on the optimal frequency of HbA1c testing [33]. The frequency depends on the clinical condition of the patients. In stable, well-controlled situations, the frequency can be lower than that for patients with poor control. The ADA recommends at least two tests a year for patients who meet treatment goals (and who have stable glycemic control), and quarterly tests in patients whose therapy has changed or who do not meet glycemic goals. In addition, all patients with diabetes who are admitted to a hospital should have their HbA1c concentrations measured if the test results of the previous 2-3 months are unavailable [27].
Although standard interpretation norms are applicable, any result should be interpreted with caution because of uncertainty. Additionally, specific clinical conditions and individualized targets for therapy should be considered.
The uncertainty of laboratory test results is often underestimated, especially by clinicians. Patient-related uncertainty stems from non-glucose-related biological variation. For HbA1c values, the major factor is erythrocyte lifespan. In normal individuals, erythrocytes survive for approximately 120 days. An HbA1c value of 43 mmol/mol (6.1%) implies a high risk for diabetes in cases where the erythrocytes have a relatively short lifespan and a low risk for diabetes in cases where erythrocytes have a relatively long lifespan. Test-related uncertainty stems from analytical errors in the test: systematic bias due to non-ideal calibration and random bias because of imprecision [6]. Interpretation uncertainty is the sum of patient- and test-related uncertainty. Because of the small margins in clinical decision limits, uncertainty should always be taken into account, especially when the HbA1c concentration is used for diagnosis.
The major advantage of HbA1c is the lack of impact of fluctuating glucose after meals and with illness. However, several conditions should be considered. In clinical conditions where the erythrocyte lifespan is substantially shorter (e.g., renal anemia with use of erythropoietin, chronic and hemolytic anemia, acute blood loss, and recent transfusion), results will show a false, low-level HbA1c. Liver disease, dialysis, and chronic malaria may also cause a false, low-level HbA1c. Iron deficiency anemia may cause a false, high-level HbA1c because of (assumed) altered glycation rates [14, 21]. The impact of age and race is currently under discussion. Some studies show that the HbA1c concentration increases by approximately 1 mmol/mol (0.1%) per decade [34]. Other studies suggest that the HbA1c concentration is higher in US African Americans and Hispanic populations than in Caucasians, but there are no definite conclusions. It is also unclear whether this would have clinical significance [21]. Although it is assumed that reference ranges and decision limits for Asians are similar to those for Caucasians, this has not been confirmed in reliable epidemiological studies in Indo-Asian and Sino-Asian populations.
Targets are derived from the balance of minimum long-term complications, minimum risk of hypoglycemia, and quality of life. The longer is the life expectancy, the greater is the chance of long-term complications. More stringent targets for therapy will therefore be beneficial. If the life expectancy is low, the quality of life should be the priority, and overly strict regimens should be avoided. Lower targets can also be considered for patients with a short duration of diabetes and the absence of cardiovascular disease, and in type II diabetes patients on a diet. Higher targets are indicated for individuals with advanced vascular complications or a high frequency of hypoglycemia, as well as for children and adolescents [27]. Efforts to achieve an extremely low HbA1c concentration are controversial; apart from the increased frequency of hypoglycemic episodes, clinical conditions such as macrovascular diseases do not decrease or even increase with a lower HbA1c concentration [21].
Since the landmark clinical trials (DCCT, UKPDS) clearly demonstrated the relationship between glycemic control, HbA1c, and diabetic complications, the test has greatly improved, thanks to the IFCC/NGSP standardization and the ongoing efforts of the diagnostic industry. The HbA1c concentration is now a reliable test and indispensable tool in both the routine management and diagnosis of diabetes. However, global availability and accessibility of adequate assays, with traceability to the IFCC-RMP have not yet been achieved, especially in developing countries. The IFCC-RMP has been adopted as the only valid anchor for standardization, but the HbA1c concentration is still reported in different units; universal reporting therefore remains a challenge. Although the HbA1c concentration is being more frequently used for diagnosis, the degree to which biological variation limits its application needs to be resolved. Further refined reference values and clinical decision limits related to age, ethnicity, and specific patient groups need to be developed. The use of POC instruments for diagnosis is controversial. Only a few devices meet the acceptable performance criteria and how the quality of the test will work in the hands of non-professionals is questionable [35]. No objective external information is available as long as participation in EQA programs is not mandatory. Improvement of the tests and the introduction of a quality concept to warrant acceptable performance by non-laboratory staff is a challenge. Although a higher number of and better tools for the management of diabetes are now available, there is still much to be learned by clinicians, patients, and laboratory technicians, i.e., how to use these tools and how HbA1c measurements can be utilized to achieve better patient care.
References
1. World Health Organization. WHO diabetes media centre. Updated on Mar 2013. http://www.who.int/mediacentre/factsheets/fs312/en/index.html.
2. American Diabetes Association. Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008; 31:596–615. PMID: 18308683.
3. . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993; 329:977–986. PMID: 8366922.
4. . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:837–853. PMID: 9742976.
5. White NH, Sun W, Cleary PA, Tamborlane WV, Danis RP, Hainsworth DP, et al. Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents. Diabetes. 2010; 59:1244–1253. PMID: 20150283.

6. Weykamp C, John WG, Mosca A. A review of the challenge in measuring hemoglobin A1c. J Diabetes Sci Technol. 2009; 3:439–445. PMID: 20144280.

7. Jeppsson JO, Kobold U, Barr J, Finke A, Hoelzel W, Hoshino T, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002; 40:78–89. PMID: 11916276.

8. Hoelzel W, Weykamp C, Jeppsson JO, Miedema K, Barr JR, Goodall I, et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004; 50:166–174. PMID: 14709644.

9. Jaisson S, Leroy N, Meurice J, Guillard E, Gillery P. First evaluation of Capillarys 2 Flex Piercing (Sebia)® as a new analyzer for HbA1c assay by capillary electrophoresis. Clin Chem Lab Med. 2012; 50:1769–1775. PMID: 23089707.

10. Mallia AK, Hermanson GT, Krohn RI, Fujimoto EK, Smith PK. Preparation and use of a boronic acid support for separation and quantitation of glycosylated hemoglobins. Anal Lett. 1981; 14:649–661.
11. John WG, Gray MR, Bates DL, Beacham JL. Enzyme immunoassay--a new technique for estimating haemoglobin A1c. Clin Chem. 1993; 39:663–666. PMID: 8472363.
12. Liu L, Hood S, Wang Y, Bezverkov R, Dou C, Datta A, et al. Direct enzymatic assay for % HbA1c in human whole blood samples. Clin Biochem. 2008; 41:576–583. PMID: 18261468.
13. Weykamp CW, Penders TJ, Muskiet FA, van der Slik W. Influence of hemoglobin variants and derivatives on glycohemoglobin determinations, as investigated by 102 laboratories using 16 methods. Clin Chem. 1993; 39:1717–1723. PMID: 7689046.

14. Little RR, Rohlfing CL. The long and winding road to optimal HbA1c measurement. Clin Chim Acta. 2013; 418:63–71. PMID: 23318564.

15. NGSP. HbA1c assay interferences. Updated on Jul 2013. http://www.ngsp.org/interf.asp.
16. Greg Miller W, Myers GL, Lou Gantzer M, Kahn SE, Schönbrunner ER, Thienpont LM, et al. Roadmap for Harmonization of clinical laboratory measurement procedures. Clin Chem. 2011; 57:1108–1117. PMID: 21677092.

17. Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE. NGSP Steering Committee. The national glycohemoglobin standardization program: a five-year report. Clin Chem. 2001; 47:1985–1992. PMID: 11673367.
18. Shima K, Endo J, Oimomi M, Omori Y, Katayama Y, Kanazawa Y, et al. Interlaboratory differences in GHb measurement in Japan: the fifth report of the GHb standardization committee, the Japan Diabetes Society. J Jap Diabetes Soc. 1998; 41:317–323.
19. Jeppsson JO, Jerntorp P, Sundkvist G, Englund H, Nylund V. Measurement of hemoglobin A1c by a new liquid-chromatographic assay: methodology, clinical utility, and relation to glucose tolerance evaluated. Clin Chem. 1986; 32:1867–1872. PMID: 3757206.

20. Weykamp C, John WG, Mosca A, Hoshino T, Little R, Jeppsson JO, et al. The IFCC Reference Measurement System for HbA1c: a 6-year progress report. Clin Chem. 2008; 54:240–248. PMID: 18223132.

21. Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011; 57:e1–e47. PMID: 21617152.

22. Consensus Committee. Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007; 30:2399–2400. PMID: 17726190.
23. Little RR, Rohlfing CL, Tennill AL, Connolly S, Hanson S. Effects of sample storage conditions on glycated hemoglobin measurement: evaluation of five different high performance liquid chromatography methods. Diabetes Technol Ther. 2007; 9:36–42. PMID: 17316096.

24. Little RR, Rohlfing CL, Sacks DB. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011; 57:205–214. PMID: 21148304.

25. Weykamp CW, Mosca A, Gillery P, Panteghini M. The analytical goals for hemoglobin A(1c) measurement in IFCC units and National Glycohemoglobin Standardization Program Units are different. Clin Chem. 2011; 57:1204–1206. PMID: 21571810.

26. American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care. 2011; 34(S1):S11–S61. PMID: 21193625.
27. American Diabetes Association. Standards of medical care in diabetes-2010. Diabetes Care. 2010; 33(S1):S11–S61. PMID: 20042772.
28. John WG. UK Department of Health Advisory Committee on Diabetes. Use of HbA1c in the diagnosis of diabetes mellitus in the UK. The implementation of World Health Organization guidance 2011. Diabet Med. 2012; 29:1350–1357. PMID: 22957983.
29. Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest. 2010; 1:212–228.

30. WHO. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. http://www.who.int/diabetes/publications/report-hba1c_2011.pdf.
31. Kitzmiller JL, Block JM, Brown FM, Catalano PM, Conway DL, Coustan DR, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care. 2008; 31:1060–1079. PMID: 18445730.
32. Davidson MB. Diabetes research and diabetes care. Where do we stand? Diabetes Care. 1998; 21:2152–2160. PMID: 9839110.

33. Larsen ML, Hørder M, Mogensen EF. Effect of long-term monitoring of glycosylated hemoglobin levels in insulin-dependent diabetes mellitus. N Engl J Med. 1990; 323:1021–1025. PMID: 2215560.

34. Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, et al. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010; 152:770–777. PMID: 20547905.
35. Lenters-Westra E, Slingerland RJ. Six of eight haemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010; 56:44–52. PMID: 19926777.
Fig. 1
Analytical concepts of HbA1c measurement methods and their traceability to the IFCC-RMP.
Abbreviations: IFCC, International Federation of Clinical Chemistry; RMP, Reference Measurement Procedure.
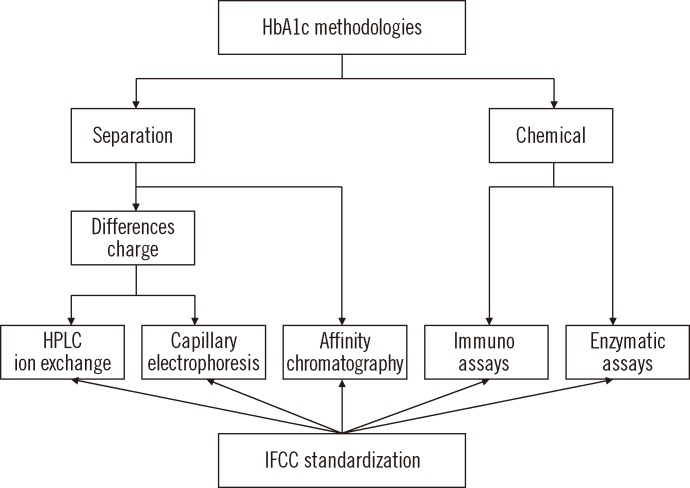
Fig. 2
Quality chain of IFCC-RMP-standardized HbA1c testing. The manufacturer uses calibrators, to which values have been assigned with the IFCC-RMP, to assign values to the kit calibrators. The EQA/PT provider uses samples also targeted by the IFCC-RMP. Good performance of the whole chain is demonstrated when the laboratory (clinical chemist), using the kit calibrators of the manufacturer, measures the correct HbA1c values in EQA/PT samples. Subsequently, all results of patients are traceable to the IFCC-RMP, and diabetologists can use universal reference values and decision limits.
Abbreviations: IFCC-RMP, International Federation of Clinical Chemistry Reference Measurement Procedure; EQA, external quality assessment; PT, proficiency testing.
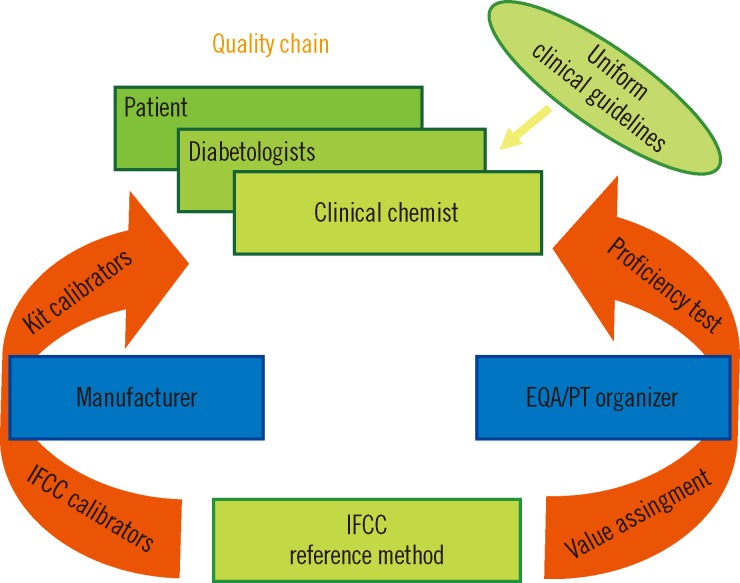
Fig. 3
The global distribution of reference laboratories operating the IFCC-RMP.
Abbreviations: IFCC-RMP, International Federation of Clinical Chemistry Reference Measurement Procedure; US, the Unites States; BR, Brazil; NL, the Netherlands; D, Germany; F, France; I, Italy; IN, India; CN, China; KO, Korea; JP, Japan.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download