Abstract
Purpose
This study aimed to evaluate the prevalence of Haller cells and accessory maxillary ostium (AMO) in cone-beam computed tomography (CBCT) images, and to analyze the relationships among Haller cells, AMO, and maxillary sinusitis.
Materials and Methods
Volumetric CBCT scans from 201 patients were retrieved from our institution's Digital Imaging and Communications in Medicine archive folder. Two observers evaluated the presence of Haller cells, AMO, and maxillary sinusitis in the CBCT scans.
Results
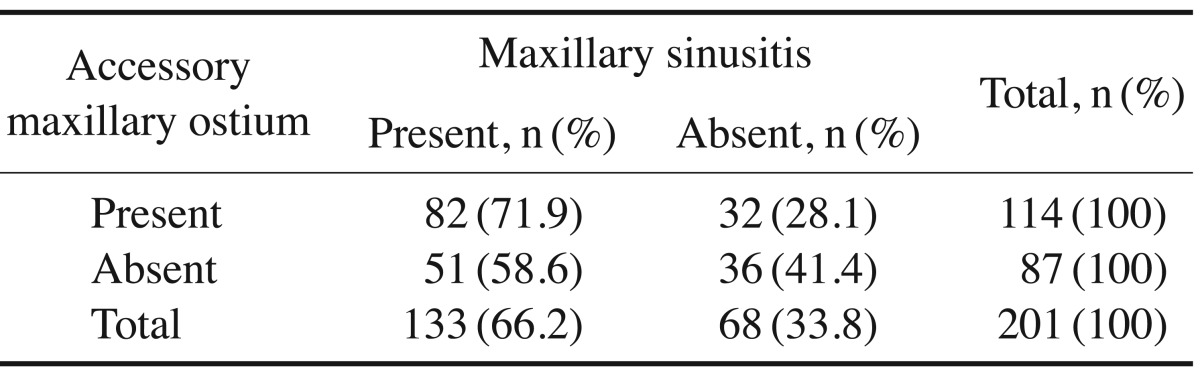
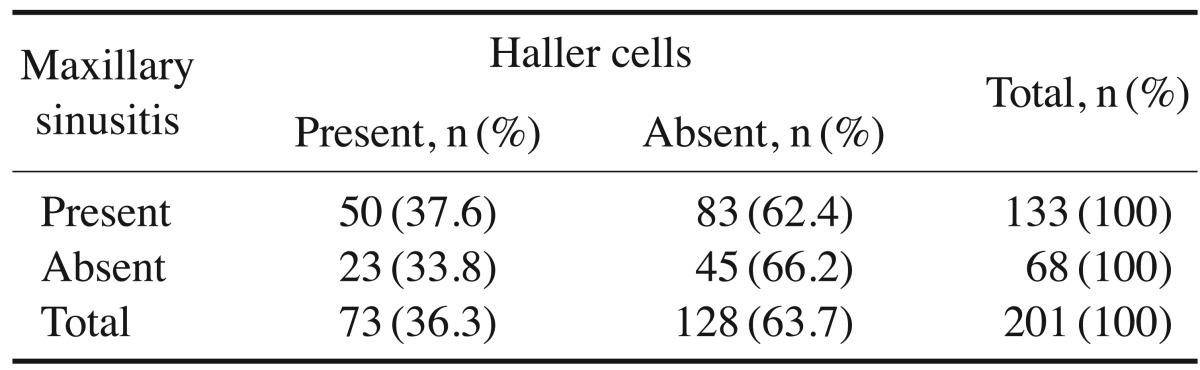
AMO was observed in 114 patients, of whom 27 (23.7%) had AMO exclusively on the right side, 26 (22.8%) only on the left side, and 61 (53.5%) bilaterally. Haller cells were identified in 73 (36.3%) patients. In 24 (32.9%) they were present exclusively on the right side, in 17 (23.3%) they were only present on the left side, and in 32 (43.8%) they were located bilaterally. Of the 73 (36.3%) patients with Haller cells, maxillary sinusitis was also present in 50 (68.5%). On using chi-square test, a significant association was observed between AMO and maxillary sinusitis in the presence of Haller cells.
The increasing recognition of functional endoscopic sinus surgery has led to the emergence of interest in the complex radiological anatomy of the paranasal sinus region. Anatomic reviews in the recent literature have often focused on subtle variations. However, variations that were once solely the preserve of the anatomist can now be satisfactorily imaged using 3-dimensional imaging techniques.12 Accessory maxillary ostium (AMO) is an anatomic variant that may play a role in the development of maxillary sinusitis.3 Although some investigators maintain that the accessory ostium develops after acute maxillary sinusitis, it is still not established whether AMO is a congenital or an acquired structure.4 Genc et al.4 investigated the development of accessory ostium and confirmed that AMO developed following experimentally induced sinusitis. The prevalence of AMO has been found to be higher in patients with a history of infundibular obstruction or maxillary sinus infection,567 suggesting that AMO develops as a result of maxillary sinusitis.
It has been well established that some anatomical variations in the paranasal sinus can predispose individuals to sinus infection or even complicate sinus surgery, and Haller cells are no exception. Haller cells are often cited as an incidental finding, without detailed investigation into their potential role in the development of sinus pathologies.8 The possible obstructive role of Haller cells in sinus drainage and their role in sinusitis, which may result in the development of AMO, has prompted investigations to assess possible associations between these anatomical variants.91011 Cadaveric and clinical investigations have confirmed the applicability of CBCT imaging in endoscopic sinus surgery, concluding that both spatial and soft-tissue contrast were satisfactory to aid surgical evaluation and navigation in the sinonasal cavity.121314
This study aimed to evaluate the prevalence of Haller cells and AMO in CBCT images, and to analyze the relationship between the presence of Haller cells and/or AMO and maxillary sinusitis.
This study received ethical approval from the institutional review board. CBCT scan records from March 2015 to February 2016 were retrieved from our institution's Digital Imaging and Communications in Medicine (DICOM) archive folder. The CBCT scans of 201 patients were included in the study. The exclusion criteria were patients who had any sinus or perisinus pathology or who had undergone surgery in the sinonasal region. CBCT scans with partially reconstructed images and artifacts compromising the diagnostic quality of the scans were also excluded.
CBCT scans were obtained using the Kodak CS 9300 3D system (Carestream Health Inc., Rochester, NY, USA) with a 17×13.5-cm field of view, a 250×250×250-µm voxel size, 70 kVp, 10 mA, and an X-ray pulse time of 30 ms. Observers assessed the reconstructed images in the axial, sagittal, and coronal planes using the DICOM format.
Precise criteria were used to recognize Haller cells as air cells of any size located medial to the infraorbital foramen on the orbital floor and roof of maxillary sinus, above the maxillary sinus ostium and within the ethmoid infundibulum. Maxillary sinusitis was defined as radiographic mucosal thickening and/or fluid accumulation at any level. The presence of mucous retention was not considered to be a form of sinus pathology. On CBCT, mucous retention phenomena are radiopaque, dome-shaped structures with a rounded edge, located on the floor of the maxillary sinus; additionally, mucosal and cortical integrity are preserved, unlike mucosal thickening or fluid accumulation. AMO was considered to be any opening other than the primary ostium located below the uncinated process and above the inferior turbinates along the medial wall of the maxillary sinus.
A session was arranged for observers before commencement of the study to train them in precisely identifying Haller cells, AMO, and maxillary sinusitis. Two observers, both skilled and experienced radiologists with at least 6 years of experience in reading CBCT scans, assessed the scans individually at different times. When disagreements occurred, the observers assessed the CBCT images together until a consensus was reached. All CBCT scans were in the DICOM format and were transferred to another computer. The CBCT images were assessed using the Kodak Digital image communication software (version 6.12.10.0, Carestream Health Inc., Rochester, NY, USA) on a workstation with a 19-inch HP LE 1911 LCD display (Hewlett Packard Company, Palo Alto, CA, USA) with a resolution of 1280×1024 pixels. Observers were free to use the contrast tool, and all observations were made in a dimly lit room. The observers were asked to assess the presence of Haller cells, AMO, and maxillary sinusitis.
All data was transferred to an Excel spreadsheet (Microsoft Corp., Redmond, WA, USA), and a descriptive statistical analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The chi-square test was used to compare the associations among Haller cells, AMO, and maxillary sinusitis. Intraobserver variability was calculated using Fleiss and Cohen kappa statistics. Interobserver agreement was evaluated using Fleiss kappa statistics.
Of the 201 patients included in the study, 104 were female and 97 were male, and they ranged in age from 16 to 85 years (mean, 37 years).
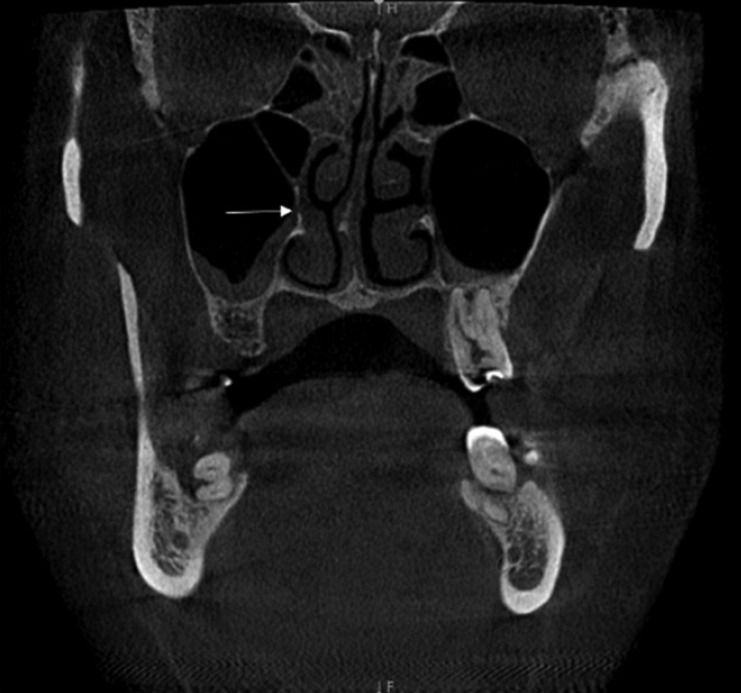
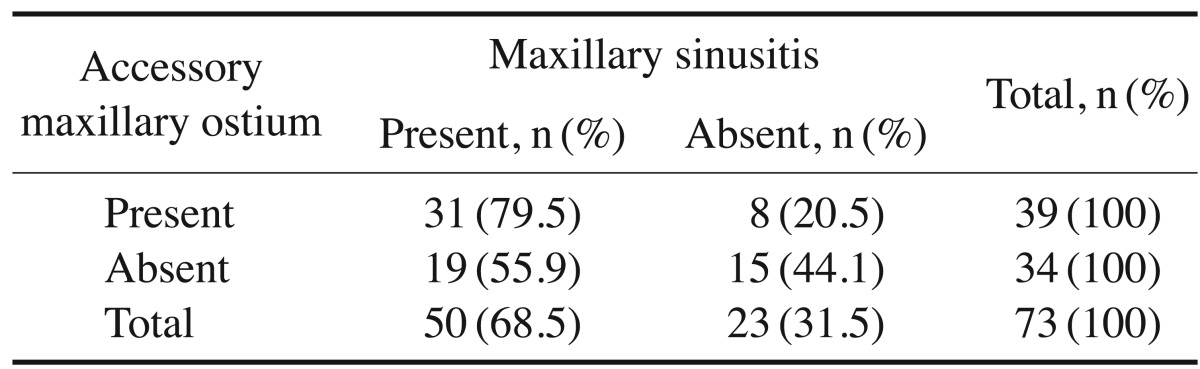
AMO was observed in 114 patients. In 27 of these patients (23.7%), AMO was present exclusively on the right side (Fig. 1), in 26 (22.8%) only on the left side, and in 61 (53.5%) bilaterally. Of these patients, 71.9% had both AMO and maxillary sinusitis. The chi-square test demonstrated a significant association between the presence of AMO and maxillary sinusitis (P=.04) (Table 1).
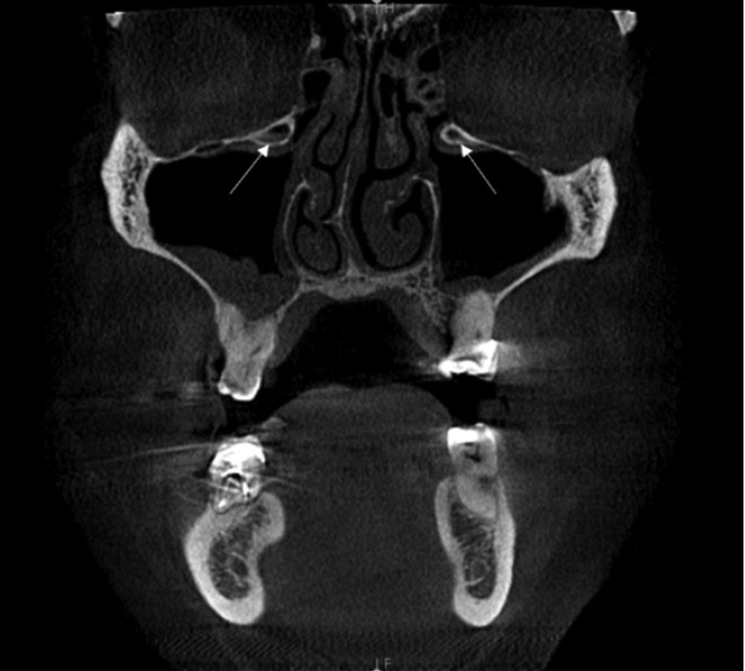
Haller cells were recognized in 73 patients (36.3%). They were present exclusively on the right side in 24 patients (32.9%), only on the left side in 17 patients (23.3%), and bilaterally in 32 patients (43.8%) (Fig. 2). Maxillary sinusitis was noted in 66.2% (n=133) of the total sample of patients. Of the patients with Haller cells, 37.6% demonstrated maxillary sinusitis. The chi-square test demonstrated no significant association between the presence of Haller cells and maxillary sinusitis (P=.599) (Table 2).
The chi-square test showed a significant association between AMO and maxillary sinusitis in the presence of Haller cells (P=.03) (Table 3).
Interobserver agreement was analyzed using Fleiss kappa statistics. The kappa score indicated almost perfect agreement (0.894). The kappa score for intraobserver agreement was 0.904.
A literature search did not reveal any previous studies investigating the prevalence and clinical significance of AMO using CBCT in human subjects. To the best of our knowledge, this was the first study that used CBCT to analyze the prevalence of AMO and its association with Haller cells and maxillary sinusitis. Earlier cadaveric and clinical examinations have reported the prevalence of AMO in humans to range from 0% to 43%.1516 Yenigun et al.3 performed a study in which AMO was detected in a total of 72 (19.1%) patients. In the patients with AMO, it was located on the right side in 7.2% of the entire sample, on the left side in 3.7%, and bilaterally in 8.2%; a statistically significant association was observed between the occurrence of AMO and maxillary sinusitis. Earwaker17 performed a study investigating anatomical variants in sinonasal computed tomography, and reported a 14% prevalence of AMO. May et al.18 reported a 10% prevalence of AMO in patients who underwent sinus surgery, but did not find AMO in any of the cadavers included in their study. Mladina et al.5 noted that the prevalence of AMO was higher in patients suffering from maxillary sinusitis (19.9%) than in healthy volunteers (0.48%). In the present study, AMO was found in 23.7% of patients, and a statistically significant association was observed between the presence of AMO and maxillary sinusitis.
The prevalence of Haller cells in the literature has been reported to be extremely variable, ranging from 2.7% to 45.1%.1920 This variability may be attributed to variation in subjects' age and race, and in the imaging techniques used.21 In the present study, we generated our own criteria for defining the presence of Haller cells based primarily on their position. The prevalence of Haller cells in our study was in fair agreement with earlier studies (36.3%). In the present study, several of the Haller cells that we recognized were less than 1 mm in size; such anatomical landmarks might likely to be missed in multislice CT (MSCT). Since CBCT has a higher spatial resolution than MSCT, it captures any Haller cell when present, irrespective of size. The high prevalence of Haller cells in our study may indicate the sensitivity of CBCT scans for the recognition of small delicate bony structures. This observation offers evidence of the usefulness of CBCT in the accurate imaging of bony structures of the orbit at a substantially lower radiation dose.
In a study performed by Genc et al.,4 experimental sinusitis was induced in the right sides of 5 rabbits. Following sacrifice, the lateral nasal walls were examined for the development of AMO, which developed in 2 of the 5 sides with sinusitis (40%), establishing that AMO developed following experimental sinusitis in rabbits. Several previous studies have confirmed the association of Haller cells with maxillary sinusitis.91011 However, in our study, no significant association was found between Haller cells and maxillary sinusitis, although a significant association was observed between AMO and sinusitis in the presence of Haller cells. This could be explained on the basis of the findings of Genc et al. that AMO developed in rabbits following induced sinusitis. Additionally, it has been well documented in the literature that Haller cells can obstruct the opening of the maxillary sinus and disturb the mucociliary flow, causing persistent recurrent sinusitis.91011 For this reason, we suggest that Haller cell-induced sinusitis can result in the development of AMO. The major limitation of our study is that the prevalence of maxillary sinusitis could have been overestimated since it was not possible to differentiate between infectious sinusitis and allergic sinusitis based on radiographic assessments alone.
In conclusion, our analysis showed that AMO and Haller cells were associated with maxillary sinusitis. This study provided evidence for the usefulness of CBCT in imaging the bony anatomy of the sinonasal complex with significantly higher precision and less radiation. Further CBCT assessment of patients with definite maxillary sinusitis is strongly recommended to investigate the associations of AMO and Haller cells with maxillary sinusitis.
References
2. Lloyd GA. CT of the paranasal sinuses: study of a control series in relation to endoscopic sinus surgery. J Laryngol Otol. 1990; 104:477–481. PMID: 2376707.

3. Yenigun A, Fazliogullari Z, Gun C, Uysal II, Nayman A, Karabulut AK. The effect of the presence of the accessory maxillary ostium on the maxillary sinus. Eur Arch Otorhinolaryngol. 2016; 273:4315–4319. PMID: 27300297.

4. Genc S, Ozcan M, Titiz A, Unal A. Development of maxillary accessory ostium following sinusitis in rabbits. Rhinology. 2008; 46:121–124. PMID: 18575013.
5. Mladina R, Vuković K, Poje G. The two holes syndrome. Am J Rhinol Allergy. 2009; 23:602–604. PMID: 19958610.

6. Kane KJ. Recirculation of mucus as a cause of persistent sinusitis. Am J Rhinol. 1997; 11:361–369. PMID: 9768318.

7. Gutman M, Houser S. Iatrogenic maxillary sinus recirculation and beyond. Ear Nose Throat J. 2003; 82:61–63. PMID: 12610908.

8. Mathew R, Omami G, Hand A, Fellows D, Lurie A. Cone beam CT analysis of Haller cells: prevalence and clinical significance. Dentomaxillofac Radiol. 2013; 42:20130055. PMID: 23975112.

9. Stammberger H, Wolf G. Headaches and sinus disease: the endoscopic approach. Ann Otol Rhinol Laryngol Suppl. 1988; 134:3–23. PMID: 3140703.

10. Stackpole SA, Edelstein DR. The anatomic relevance of the Haller cell in sinusitis. Am J Rhinol. 1997; 11:219–223. PMID: 9209594.

11. Kantarci M, Karasen RM, Alper F, Onbas O, Okur A, Karaman A. Remarkable anatomic variations in paranasal sinus region and their clinical importance. Eur J Radiol. 2004; 50:296–302. PMID: 15145491.

12. Rafferty MA, Siewerdsen JH, Chan Y, Moseley DJ, Daly MJ, Jaffray DA, et al. Investigation of C-arm cone-beam CT-guided surgery of the frontal recess. Laryngoscope. 2005; 115:2138–2143. PMID: 16369157.

13. Jackman AH, Palmer JN, Chiu AG, Kennedy DW. Use of intraoperative CT scanning in endoscopic sinus surgery: a preliminary report. Am J Rhinol. 2008; 22:170–174. PMID: 18416975.

14. Batra PS, Kanowitz SJ, Citardi MJ. Clinical utility of intraoperative volume computed tomography scanner for endoscopic sinonasal and skull base procedures. Am J Rhinol. 2008; 22:511–515. PMID: 18954511.

15. Kumar H, Choudhry R, Kakar S. Accessory maxillary ostia: topography and clinical application. J Anat Soc India. 2001; 50:3–5.
16. Jog M, McGarry GW. How frequent are accessory sinus ostia? J Laryngol Otol. 2003; 117:270–272. PMID: 12816215.

18. May M, Sobol SM, Korzec K. The location of the maxillary os and its importance to the endoscopic sinus surgeon. Laryngoscope. 1990; 100:1037–1042. PMID: 2215032.

19. Bolger WE, Butzin CA, Parsons DS. Paranasal sinus bony anatomic variations and mucosal abnormalities: CT analysis for endoscopic sinus surgery. Laryngoscope. 1991; 101:56–64. PMID: 1984551.
20. Pérez-Piñas I, Sabaté J, Carmona A, Catalina-Herrera CJ, Jiménez-Castellanos J. Anatomical variations in the human paranasal sinus region studied by CT. J Anat. 2000; 197:221–227. PMID: 11005714.
21. Rysz M, Bakoń L. Maxillary sinus anatomy variation and nasal cavity width: structural computed tomography imaging. Folia Morphol (Warsz). 2009; 68:260–264. PMID: 19950077.
Fig. 1
A coronal cone-beam computed tomography image demonstrates accessory maxillary ostium in the right maxillary sinus with ipsilateral maxillary sinusitis (white arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download