Abstract
Necrotizing sialometaplasia (NS) which mimics malignancy both clinically and histopathologically is an uncommon benign, self-limiting inflammatory disease of the mucus-secreting minor salivary glands. The lesion is believed to be the result of vascular ischemia that may be initiated by trauma. Till date, the diagnosis of NS remains a challenge. This report demonstrates a case of NS in a 73-year-old male patient who presented with an ulcerative lesion in his palate. He had a history of local trauma and was long-term user of salbutamol inhaler. An incisional biopsy was carried out and the diagnosis was established through history, clinical examination, histopathology using Hematoxylin and Eosin stain. The patient was given symptomatic treatment and the lesion healed in about 7 weeks.
Necrotizing sialometaplasia (NS) is a benign, self limiting inflammatory reaction of salivary gland tissue which may mimic squamous cell carcinoma or mucoepidermoid carcinoma both clinically and histologically and can lead to unnecessary surgical approach.1 NS was first reported in 1973 by Abrams et al2 as a reactive necrotizing inflammatory process involving minor salivary gland of the hard palate. In the WHO classification of salivary gland tumors, NS is classified under the group of tumor-like lesions. NS is extremely rare; only about 200 cases have been reported in world literature.4 Diagnosis of NS still remains a challenge. Mesa et al1 suggested that NS occurred in only 0.03% of all biopsied oral lesions. This condition shows a white predominance and could occur in any age group ranging from 17 to 80 years of age with a mean age of 50 years in men and 36 years in women with a male predominance of 2 : 1.3
It most commonly affects the minor salivary glands of the palate (80%).4 Other sites include retromolar pad, gingiva, lip, tongue, cheek, nasal cavity, sinuses, larynx, and trachea where the salivary gland tissue is located.3,5-10 The lesion has also been known to occur at extra salivary sites including lungs,11 breast,12 and skin. In the previous reports, the lesion occurring outside the salivary glands, was designated as adenometaplasia9 and when skin was affected, by this particular lesion it was termed as syringometaplasia or metaplasia of sweat ducts.13
This report presents a case of NS in a 73-year-old male who was a long-time user of sulbutamol inhaler and had a history of local trauma.
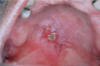
A 73-year-old male patient presented at the Department of Oral Medicine and Radiology with ulcerative lesion in his palate. The lesion was discovered by his dentist during a routine check-up. The patient himself was unaware of the lesion. He gave a history of pressing soft biscuits on his palate while eating, 2-3 weeks prior to the occurrence of the lesion. The ulcer was not associated with pain or paresthesia. About 10 years before he was treated for adenocarcinoma of rectum and tuberculosis of abdomen. He was a known asthmatic and was on salbutamol inhalation. On intraoral examination, two irregular shaped ulcers were noticed in the posterior part of the palate (junction of hard and soft palate), one measuring about 1.5×1 cm and the other measuring about 0.8×0.5 cm. The margins of the ulcers were slightly raised, erythematious with surrounding mucosa appearing blanched, pale and grayish white. The underlying bone was partially exposed, the floor was covered by yellowish grey slough, and the base was mildly indurated (Fig. 1). As the patient had a previous history of tuberculosis; a provisional diagnosis of tuberculous ulcer of palate was made. Differential diagnosis of necrotizing sialometaplasia of minor salivary gland of palate, chronic traumatic ulcer, malignant ulcer, both primary and secondary from adenocarcinoma of rectum, syphilitic ulcer, adenocarcinoma of minor salivary gland of palate were considered.
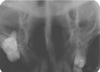
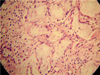
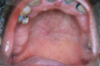
Occlusal radiograph (Fig. 2) showed no bony involvement. Chest radiograph and blood investigations also demonstrated normal state, and rapid plasma regain for syphilis was negative. Incisional biopsy was performed and histopathologic features on hematoxylin-eosin stain were suggestive of NS (Figs. 3 and 4). The patient was advised to apply gentian violet 2-3 times a day with cotton and maintain good oral hygiene with saline rinse 3-4 times a day. The lesion showed remission after 6 weeks of follow-up except for an area of erythema (Fig. 5) which resolved completely after a week.
NS is defined as squamous metaplasia of the salivary gland ducts and acini with ischemic necrosis of the salivary gland lobules and it occurs at hard palate most frequently. The pathogenesis is unknown but it is believed to be due to ischemia of vasculature supplying the salivary gland lobules. Etiological factors for ischemia can be direct trauma, administration of local anesthetic, ill fitting dentures, alcohol, smoking and cocaine use, radiation, incubation and surgical procedures and upper respiratory infections.14 Nah et al15 reported a case involving a 59-year-old male patient who developed a NS following excisional biopsy of adenoid cystic carcinoma. It was hypothesized that in our patient, pressing of biscuits on the palate, lead to ischemia, which resulted in infarction and ulcer of the salivary tissues. This could have been further aggravated by his long-term use of salbutamol which might have led to dehydration of the mucosa, thinning of the mucosa, thus making it more susceptible to local trauma.
In most cases, NS occurs spontaneously and the initial symptoms may vary. These include fever, chills, malaise, or swelling.3 Majority of cases of NS occurred at the posterior hard palate. The junction of the hard and soft palate was the second most common site. About two thirds of the palatal lesions were unilateral; however, bilateral synchronous and metachronus lesions were not uncommon. Also the lesion could occur in the midline.16,17 The size ranged from 0.7 to 5.0cm (average 1.8 cm).15 Typically it presented as a crateriform ulcer of the palate that simulates malignant process. In some cases, subacute variant might present as a rapidly growing swelling. Lesions were usually painless but some cases associated with pain had also been reported. Anesthesia of the palatal mucosa was reported as an early indicator of this form of ulcerations.18 Keogh et al17 reported a case of unusual NS which presented as bilateral palatal swelling associated with complete anesthesia of the palate. Lesions were usually shallow; the margins of the ulcer were often everted and indurated resembling a carcinoma. Cases with palatal bone erosion might occur in both ulcerated and non ulcerated cases though it was not evident in our case. Radiographically, most of NSs showed no bony involvement except for few cases showing saucerisation of the underlying palatal bone.3
The appearance of the ulcer in our patient suggested other possible conditions like secondaries from adenocarcinoma of rectum, primary adenocarcinoma of the palate, squamous cell carcinoma, subacute necrotizing sialadenitis (SANS), major aphthous ulcer, mucoepidermoid carcinoma, secondary syphilis, and tuberculous ulcer. Chest skiagram and occlusal radiograph did not show any abnormalities. Routine haemogram was within the normal parameters and serology for syphilis was nonreactive. Incisional biopsy was performed and the histological features suggested the diagnosis of NS.
The diagnosis of NS might be difficult and is based on a complete clinical history and a well-oriented biopsy section. A combination of histopathological and clinical findings is often helpful in establishing the confirmatory diagnosis. The diagnosis can be further supplemented via immunohistochemistry demonstrating focal to absent immunoreactivity for p53, low immunoreactivity for MIB1 (Ki-67), and the presence of 4A4/p63 and calponin-positive myoepithelial cells. Nonetheless, to date, hematoxylineosin staining remains the gold standard.19 The histologic diagnostic criteria for NS are well established through a number of previous reports.2,3,20 Since the diagnostic criteria of this lesion are quite distinctive, proper care should be taken in diagnosis of this lesion, so that misdiagnosis and unnecessary and radical treatment can be avoided. Anneroth and Hansen10 described the histopathogenesis of NS by proposing five histological stages: infarction, sequestration, ulceration, repair, and healing. Histological features exhibit a spectrum ranging from ulceration, lobular necrosis, sequestration of necrotic acini, pseudoepitheliomatous hyperplasia of adjacent epithelium, squamous metaplasia of ductal epithelium, and inflammatory changes.2 Our case showed pseudoepitheliomatous hyperplasia of epithelium with acinar cell necrosis and squamous metaplasia of ductal epithelium. However the lobular architecture was maintained.
Usually no treatment is required and the lesion heals by secondary intention within 4 to 10 weeks (average 5.2 weeks). Even a full-thickness palatal lesion communicating with nasal cavity resolved completely in six months.3 In our case the lesion showed complete remission in about 7 weeks.
In conclusion, NS is a benign self-limiting disorder of salivary glands. Unfortunately, it has been misdiagnosed clinically and microscopically as a malignant neoplasm, resulting in inappropriate treatment. A simple incisional biopsy is required to confirm the histologic diagnosis and to rule out more serious disease processes. It is a self-limiting disease process and requires no treatment. The exact etiology though cannot be determined in this case the role of long term use of salbutamol inhaler as a predisposing factor needs to be assessed.
Figures and Tables
Fig. 1
The clinical photograph shows 2 irregular shaped ulcers in the posterior part of the palate with raised erythematous margins and surrounding mucosa appearing blanched, pale and grayish white. The underlying bone was partially exposed, and the floor was covered by yellowish grey slough.
References
1. Mesa ML, Gertler RS, Schneider LC. Necrotizing sialometaplasia: frequency of histologic misdiagnosis. Oral Surg Oral Med Oral Pathol. 1984. 57:71–73.

2. Abrams AM, Melrose RJ, Howell F. Necrotizing sialometaplasia. A disease simulating malignancy. Cancer. 1973. 32:130–135.

3. Brannon RB, Fowler CB, Hartman KS. Necrotizing sialometaplasia. A clinicopathologic study of sixty-nine cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1991. 72:317–325.
4. Schmidt-Westhausen A, Philipsen HP, Reichart PA. Necrotizing sialometaplasia of the palate. Literature report of 3 new cases. Dtsch Z Mund Kiefer Gesichtschir. 1991. 15:30–34.
5. Randhawa T, Varghese I, Shameena PM, Sudha S, Nair RG. Necrotizing sialometaplasia of tongue. J Oral Maxillofac Pathol. 2009. 13:35–37.

7. Walker GK, Fechner RE, Johns ME, Teja K. Necrotizing sialometaplasia of the larynx secondary to atheromatous embolization. Am J Clin Pathol. 1982. 77:221–223.

8. Schöning H, Emshoff R, Kreczy A. Necrotizing sialometaplasia in two patients with bulimia and chronic vomiting. Int J Oral Maxillofac Surg. 1998. 27:463–465.

9. Romagosa V, Bella MR, Truchero C, Moya J. Necrotizing sialometaplasia (adenometaplasia) of the trachea. Histopathology. 1992. 21:280–282.

10. Anneroth G, Hansen LS. Necrotizing sialometaplasia. The relationship of its pathogenesis to its clinical characteristics. Int J Oral Surg. 1982. 11:283–291.
11. Zschoch H. Mucus gland infarct with squamous epithelial metaplasia in the lung. A rare site of so-called necrotizing sialometaplasia. Pathologe. 1992. 13:45–48.
12. Hurt MA, Díaz-Arias AA, Rosenholtz MJ, Havey AD, Stephenson HE Jr. Posttraumatic lobular squamous metaplasia of breast. An unusual pseudocarcinomatous metaplasia resembling squamous (necrotizing) sialometaplasia of the salivary gland. Mod Pathol. 1988. 1:385–390.
13. King DT, Barr RJ. Syringometaplasia: mucinous and squamous variants. J Cutan Pathol. 1979. 6:284–291.

14. Fowler CB, Brannon RB. Subacute necrotizing sialadenitis: report of 7 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000. 89:600–609.

15. Nah KS, Cho BH, Jung YH. Necrotizing sialometaplasia: report of 2 cases. Korean J Oral Maxillofac Radiol. 2006. 36:207–209.
16. Daudia A, Murty GE. First case of full-thickness palatal necrotizing sialometaplasia. J Laryngol Otol. 2002. 116:219–220.

17. Keogh PV, O'Regan E, Toner M, Flint S. Necrotizing sialometaplasia: an unusual bilateral presentation associated with antecedent anaesthesia and lack of response to intralesional steroids. Case report and review of the literature. Br Dent J. 2004. 196:79–81.

18. Lamey PJ, Lewis MA, Crawford DJ, MacDonald DG. Necrotising sialometaplasia presenting as greater palatine nerve anaesthesia. Int J Oral Maxillofac Surg. 1989. 18:70–72.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download