Abstract
Purpose
Materials and Methods
Results
ACKNOWLEDGEMENTS
Notes
Author Contribution: Research conception & design: Park NC, Hwang SY, Park HJ, Koo YK. Performing the experiments: Park HJ, Hwang YK. Contributed new reagents or analytic tools: Hwang SY, Koo YK, Hwang YK. Data analysis and interpretation: Park HY, Park MJ. Drafting of the manuscript: Park HJ, Park NC, Park MJ.
References
Fig. 1
Histological analysis of seminiferous tubules from normal controls, luteinizing hormone-releasing hormone (LHRH)-induced infertility controls, LHRH-induced rats treated with testosterone, and LHRH-induced rats treated with 400 mg/kg of KH-465. Representative images of tubular cross-sections of the testis are shown. (A) Normal control group pretreatment, (B) normal control group after 8 weeks, (C) LHRH agonist-induced control group I after 4 weeks, (D) LHRH agonist-induced control group I after 8 weeks, (E) LHRH-induced group treated with 400 mg/kg of KH-465 (group III) after 8 weeks, and (F) LHRH-induced group treated with testosterone (group IV) after 8 weeks. All sections were stained with H&E (×400). The images are typical of those obtained in 5 independent experiments.
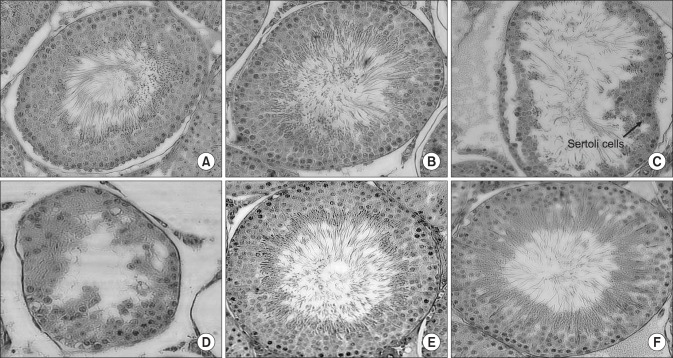
Table 1
Herb profiles of Epimedium koreanum Nakai and Angelica gigas Nakai
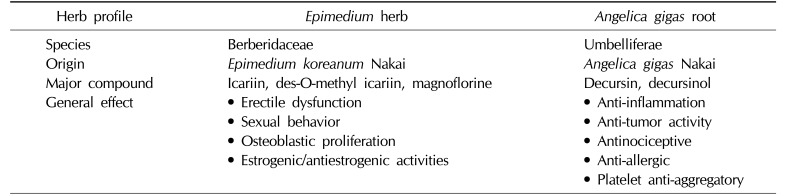
Table 2
Effects of KH-465 on the body, testis, and epididymis weights in Sprague-Dawley rats with LHRH agonist-induced infertility
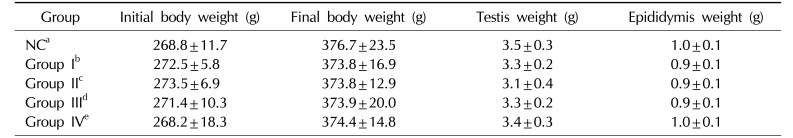
Values are presented as mean±standard deviation (of at least 3 independent experiments).
LHRH: luteinizing hormone-releasing hormone, NC: normal control.
aNormal control group, bLHRH agonist-induced infertility control group, c200 mg/kg/day of KH465 in LHRH agonist-induced infertility group, d400 mg/kg/day of KH465 in LHRH agonist-induced infertility group, e24 mg/kg/day of testosterone in LHRH agonist-induced infertility group.
Table 3
Effects of KH-465 on testicular tissue and sex hormones in Sprague-Dawley rats with LHRH agonist-induced infertility
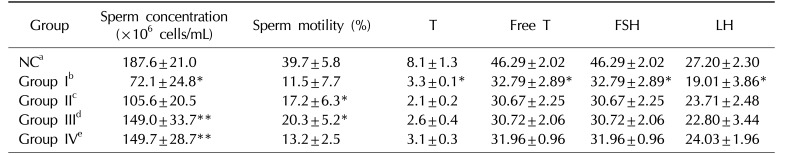
Values are presented as mean±standard deviation. *Versus NC group (p<0.05), **Versus LHRH-induced control group I (p<0.05).
LHRH: luteinizing hormone-releasing hormone, T: testosterone, FSH: follicle-stimulating hormone, LH: luteinizing hormone, NC: normal control.
aNormal control group, bLHRH agonist-induced infertility control group, c200 mg/kg/day of KH465 in LHRH agonist-induced infertility group, d400 mg/kg/day of KH465 in LHRH agonist-induced infertility group, e24 mg/kg/day of testosterone in LHRH agonist-induced infertility group.
Table 4
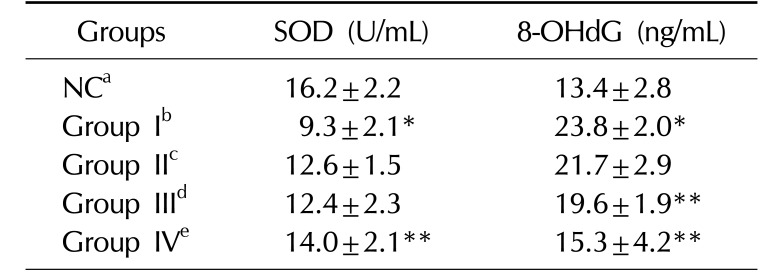
Effects of KH-465 on SOD and 8-OHdG activity in Sprague-Dawley rats with LHRH agonist-induced infertility
| Groups | SOD (U/mL) | 8-OHdG (ng/mL) |
|---|---|---|
| NCa | 16.2±2.2 | 13.4±2.8 |
| Group Ib | 9.3±2.1* | 23.8±2.0* |
| Group IIc | 12.6±1.5 | 21.7±2.9 |
| Group IIId | 12.4±2.3 | 19.6±1.9** |
| Group IVe | 14.0±2.1** | 15.3±4.2** |
Values are presented as mean±standard deviation. *Versus NC group (p<0.05), **Versus LHRH-induced control group I (p<0.05).
SOD: superoxide dismutase, 8-OHdG: 8-hydroxy-2′-deoxyguanosine, LHRH: luteinizing hormone-releasing hormone, NC: normal control.
aNormal control group, bLHRH agonist-induced infertility control group, c200 mg/kg/day of KH465 in LHRH agonist-induced infertility group, d400 mg/kg/day of KH465 in LHRH agonist-induced infertility group, e24 mg/kg/day of testosterone in LHRH agonist-induced infertility group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download