Abstract
Peyronie disease is a common cause of penile deformity and sexual dysfunction. Although surgery is regarded as the definitive management for this condition, there are many medical and minimally invasive therapies available, with widely varying efficacy reported in the literature. The purpose of this review is to describe the current state-of-the-art for each of the most commonly used as well as several developing non-surgical treatments. Further, we hope to offer perspectives that will aid practitioners in deciding among these treatments that are either already in use or have the potential to be used as alternatives to surgery in the management of this frustrating disease.
Peyronie disease (PD) is characterized as a fibrous, inelastic lesion of the tunica albuginea. It is thought to result from trauma or microtrauma to the erect penis in genetically susceptible individuals, though the mechanism of disease has not been fully elucidated [1]. The lesion can be painful in some individuals, and can also result in erection deformities making intromission difficult or impossible (Fig. 1). Disease prevalence is often quoted at 3.2% to 8.9% in adult men, though it may be higher due to underreporting. Associated comorbidities include erectile dysfunction (ED), clinical depression, diabetes, hypertension, dyslipidemia, low testosterone, obesity, smoking, and Dupuytren contractures [1,2].
The natural history of PD has two phases. First is the active (or acute) phase, characterized by active inflammation and progressive deformity of the erect penis. Penile pain, when present, also occurs at this time. The acute phase generally lasts from 6 to 18 months, and after this phase, the vast majority of patients go on to have stabilization of the plaque or progression of their disease [2]. This stabilization process is characterized by fibrosis, dystrophic calcifications, and rarely, ossification, and occurs in the chronic (or latent) phase. After the acute phase has ended, only 10% to 15% of patients will have spontaneous resolution of PD, although many will experience resolution of their associated pain [2]. Finally, the presence of intralesional calcifications is significant, as these lesions are traditionally less amenable to non-surgical treatments; PD patients with these characteristics have often been excluded from participation in non-surgical clinical trials.
The pathophysiologic basis for PD relates to the inflammation seen in the acute phase, and the subsequent disordered wound-healing characteristics of the chronic phase. Non-surgical treatment modalities to date have focused on disrupting one or both of these processes. Pluripotent stem cells within the tunica albuginea are an ongoing source of inquiry that may both help explain the disordered wound healing and shed light on novel approaches to help treat this complex disease.
We searched the literature for nonsurgical treatments of PD, including oral or topical therapy, intralesional injections (ILI), iontophoresis, extracorporeal shock wave therapy (ESWT), radiotherapy, penile traction therapy (PTT), and stem cell therapy. Aiming to build on the 2012 updates by Schaeffer and Burnett [3], and Serefoglu and Hellstrom [4], we placed emphasis on the most recent randomized controlled trials (RCTs) in order to provide the most accurate, scientifically based knowledge to guide clinical practice. See Table 1 [5-17] for a summary of recent studies, both RCTs and others, involving non-surgical treatments.
Vitamin E was first used in the treatment of PD in 1949. Its proposed mechanism of action involves scavenging free radicals that are thought to contribute to the fibrosis seen in PD [2]. Vitamin E is both inexpensive and generally considered safe. Unfortunately, these are perhaps the drug's greatest assets; in RCTs, vitamin E treatment alone appears not to be superior to a placebo [18,19]. Nonetheless, it has often been used in combination with other oral, intralesional, and iontophoresis treatments, and interestingly, it does seem to show some synergistic effect [7,9]. Since it is often given concurrently with other supplements and antioxidants, however, it is difficult to draw any conclusions on vitamin E's specific role in this synergistic effect.
Potassium para-aminobenzoate is an anti-fibrotic agent with monoamine-oxidase activity that has a long history of use in the treatment of PD [20]. In a 2005 RCT, para-aminobenzoate showed a statistically significant decrease in plaque size versus a placebo over 12 months, and seemed to confer a protective effect against the worsening of penile curvature. However, there was no statistically significant improvement in erectile curvature from baseline [21]. Further RCTs are needed to evaluate the drug's possible protective properties, but the trials are unlikely to be performed due to para-aminobenzoate's unfavorable side-effect profile (gastrointestinal upset and photosensitivity), frequent dosing (four times per day), and high cost [2].
Tamoxifen's proposed mechanism of action in PD involves modulating transforming growth factor beta (TGF-β) secretion from fibroblasts, thereby decreasing fibrogenesis within the tunica albuginea [20]. In RCTs, tamoxifen has failed to show any statistically significant benefit versus placebo [22] and was inferior to carnitine both in its efficacy and side-effect profile [23].
Best known as a treatment for gout, colchicine's proposed mechanism of action in PD stems from its anti-inflammatory properties that both decrease collagen synthesis and increase collagenase activity [20]. However, in a RCT of 84 patients, colchicine showed no superiority to a placebo over the course of 4 months of treatment [24]. Subsequent trials in combination with vitamin E showed superiority to ibuprofen monotherapy, including statistically significant improvements in plaque size and penile curvature in patients with early-stage PD [25]. Further trials are needed to help define colchicine's role in the treatment of early disease and in combination therapy.
Propionyl-L-carnitine (PLC) is short-chain acyl derivative of carnitine, an acetyl-coenzyme A inhibitor. Its role in the treatment of PD stems from its anti-oxidant properties, previously utilized for idiopathic infertility, and its antiproliferative effects on endothelial cells [19,23]. PLC has shown mixed results in the treatment of PD. In a RCT with 96 patients in 2001, it showed statistically significant improvement in penile curvature versus tamoxifen [23]. In 2002, PLC demonstrated efficacy in treating advanced and resistant PD when used in combination with verapamil ILI, which was superior to tamoxifen plus verapamil ILI therapy [26]. In the most recent RCT in 2007, however, it showed no superiority to a placebo, either alone, or in combination with concurrent vitamin E therapy [19].
Pentoxifylline (PTX) is a nonselective phosphodiesterase inhibitor with anti-inflammatory and anti-fibrogenic properties. With regard to PD, it acts to prevent TGF-β-mediated inflammation and plaque deposition of type 1 collagen [27]. In its first RCT in 2010, it was shown to improve the penile curvature, plaque area, and International Index of Erectile Function (IIEF) score versus a placebo [27]. A subsequent retrospective cohort study found significant improvement in penile calcifications over one year of treatment when compared to a placebo [15].
Phosphodiesterase type 5 (PDE5) inhibitors have long been used in the treatment of ED. In PD, these agents are proposed to inhibit tissue remodeling after acute injuries by decreasing oxidative stress responsible for inflammation and fibrosis [14]. A retrospective chart review in 2002 showed that sildenafil citrate was a safe and efficacious treatment modality for PD patients with comorbid ED [28]. Another retrospective chart review of 65 PD patients showed a statistically significant improvement in the IIEF score in those PD patients taking a low dose of tadalafil daily for six months when compared to those patients who did not take tadalafil. Of note, these PD patients were unique in that they presented with no palpable penile plaque but an isolated septal scar demonstrated by Doppler ultrasonography [14]. Finally, in the first RCT of a PDE5 inhibitor for PD, tadalafil therapy in combination with ESWT was superior to ESWT alone in both IIEF and quality of life scores [12].
Coenzyme Q10 is a fat-soluble, vitamin-like quinone that is a powerful, endogenously secreted antioxidant. Not only does it display anti-oxidant and anti-inflammatory properties itself, but it is also thought to regenerate other antioxidants within the body [29]. It is a relatively new therapy in the treatment of PD, but in its first RCT, it showed statistically significant improvement in the IIEF score, mean plaque area, and penile curvature when compared to a placebo over the course of 6 months [29].
Omega-3 fatty acids (eicosapentaenoic acid and docosahexaenoic acid) inhibit the release of inflammatory cytokines, increase collagenase activity, and compete with arachadonic acid to suppress the production of inflammatory eocosinoids [30]. In the treatment of PD, these various anti-inflammatory effects were postulated to prevent or reverse disease progression in the acute phase, but in a RCT it failed to show any significant improvement or prevention of disease progression versus a placebo [30].
First used for the treatment of PD in the 1950s, initial results with ILI of steroids were promising [1]. Subsequent trials, however, failed to reproduce these positive findings, and unfavorable side effects (including local tissue atrophy and fibrosis) made any subsequent surgical interventions more difficult [4]. For these reasons, steroid injections are no longer recommended in the treatment of PD.
Reports on the efficacy of verapamil ILI are varying. In vitro, calcium channel blockers (CCBs) such as verapamil have been shown to increase collagenase activity and decrease fibroblast proliferation [20]. In 1994, Levine et al [31] published a small nonrandomized study of escalating verapamil ILIs that demonstrated the drug's safety and efficacy in reducing plaque volumes and penile curvature. A subsequent randomized, placebo-controlled trial showed a decrease in plaque volume and a trend towards improved curvature (not statistically significant) when compared to saline ILI alone [32]. An uncontrolled trial in 2007 showed statistically significant improvement in penile rigidity in 94 patients [33], while a 2009 randomized, placebo-controlled trial with 80 patients showed no statistical significance with verapamil treatment in any of the primary outcomes (plaque size, degree of curvature, erectile function) [34].
Since then, verapamil ILI has been used in a number of trials both alone [16] and in combination with other treatment modalities (dexamethasone administered via transdermal electromotive administration [8], testosterone supplementation in low-testosterone patients with PD [17], and PTX and L-arginine given orally with or without penile traction [13]. While the number of treatment variables in these trials make it difficult to pinpoint the efficacy of verapamil ILI alone, it is worth noting that the majority of patients in all of these trials had improvement or stabilization of their disease while receiving treatment (Table 1). Finally, in its first RCT in 2010, nicardapine ILI also showed superiority to saline ILI in reducing pain and plaque size, and improving the IIEF score [35]. This suggests verapamil's role in treating PD is a class effect of CCBs independent of their being in the dihydropyridine (cardio-selective, such as verapamil) or non-dihydropyridine (vaso-selective, such as nicardapine) subset. Further trials are needed to compare the efficacy of different CCBs.
Interferons are endogenously produced cytokines that are responsible for regulating the immune response to antigenic insults, and have been shown in vitro to decrease production of fibroblasts and collagen [20]. The first two randomized trials utilizing interferon-alpha (IFN-α) ILI had contradictory results. In a trial of 30 patients (Inal et al [36], 2006) comparing intralesional IFN-α therapy alone, oral vitamin E therapy alone, or combined therapy, there was no statistical difference between groups in plaque size or degree of penile curvature. That same year, a trial with 117 patients (Hellstrom et al [37], 2006) comparing IFN-α and saline ILI showed a statistically significant improvement in penile curvature, plaque size, and plaque density in the interferon group. Finally, a single-institution, retrospective review of 127 patients treated with interferon (Trost et al [6], 2013) demonstrated a statistically significant improvement in penile curvature.
The discrepancy among these trials may be partially explained by the small sample size in the Inal trial, since the trend in the oral treatment arm was towards increasing penile curvature and plaque size, and the trend in the INF-α and combination arms were toward decreasing curvature and plaque size. The incongruent results in these trials may also be due to differences in drug dosing. The Inal trial utilized 5 million units of IFN-α ILI weekly, the Hellstrom trial utilized 5 million units every other week, and Trost's review evaluated patients receiving 2 million units every other week. Thus not only did the patients in Inal trial receive a greater cumulative dose of IFN-α, but also more frequent ILI. In a disease characterized by disordered healing, the increased tissue trauma caused by weekly injections may have offset the beneficial effects of IFN-α seen in the other trials. Further studies are needed to confirm or deny this hypothesis.
Collagenase is an enzyme that degrades interstitial collagen, making it a logical choice in the treatment of PD. First used in the treatment of PD in 1982, collagenase has not yet gained widespread acceptance. This is partially explained by the fact that collagenase is not currently approved by the U.S. FDA for the treatment of PD, though it is approved in the treatment of chronic dermal ulcers and severe burns. It has also been successfully used in the treatment of Dupuytren contractures, with which PD shares a similar pathophysiology [5].
Interest in collagenase was revived in 2008 when Jordan [38] published a study demonstrating its safety and efficacy in reducing curvature in a small population of patients. In 2013, a large multicenter Phase IIb RCT showed statistically significant improvement in penile curvature and the symptom bother domain as compared to placebo ILI [5]. Serious adverse effects occurred in approximately 1% of patients in the collagenase group, the most serious being corporeal rupture requiring surgical repair. Less serious side effects such as swelling, ecchymosis, and pain in the injection site were more common and resolved with conservative treatment. A smaller-scale trial in 2012 demonstrated a similar result, and subset analysis in that trial showed penile plaque manual modeling to be synergistic with collagenase therapy [10].
At the time of this review, the U.S. FDA has extended the Prescription Drug User Fee Act goal date for the approval of clostridial collagenase for the intralesional treatment of PD to December 6, 2013 (an extension of 3 months' time from the initial deadline). This decision is eagerly awaited by many patients and clinicians, as collagenase has shown effectiveness in a true RCT along with having been shown to be efficacious in the management of a similar disease process.
Although a topical treatment approach to PD is appealing to patients for reasons of comfort and accessibility, in practice the results are less than ideal. The greatest argument against topical therapy comes from a study of men pretreated with topical verapamil who then immediately underwent surgical correction for their PD. The excised tunica albuginea tissue samples from these PD patients were examined for the presence of verapamil, which was not seen. From this the authors concluded that with topical therapy the drug was not actually absorbed into the plaque tissue [39]. However, in the largest RCT to date there was statistically significant improvement in curvature and plaque size in patients receiving topical applications of verapamil (15% twice daily) over the course of three months [40]. The effect was statistically superior to that of a placebo, and patients who continued the topical therapy after the three-month trial continued to show improvement throughout the nine months they were followed. Further studies are needed to reconcile these contradictory findings.
Iontophoresis, or transdermal electromotive drug administration, is a method theorized to provide superior tissue penetration for the transdermal application of medications. Two trials in 2000 showed very promising results, utilizing a number of different pharmacologic agents including dexamethasone, lidocaine, orgotein, and verapamil in various combinations. Significant reductions in plaque size, curvature, and pain were found over the course of short, three-week treatment regimens [41,42]. These results were subsequently reinforced by a study of post-operative tunica albuginea tissue in patients pre-treated with iontophoresis that demonstrated a detectable level of the drug in the majority of patients [43]. In 2004, a RCT comparing verapamil and dexamethasone combination therapy to a placebo (lidocaine) demonstrated the superiority of the former, suggesting that verapamil and dexamethasone themselves had some therapeutic effect [44].This assertion was immediately called into question by a subsequent RCT that demonstrated verapamil alone had no superiority to a placebo (saline) [45]. Most recently, an unblinded RCT of 60 patients suggested that verapamil administered with iontophoresis had better results than verapamil administered intralesionally [8]. Again, further large-scale studies are needed to validate these findings.
Other noninvasive methods of treating PD include ESWT and PTT. Radiation therapy is another treatment modality occasionally described in the PD literature, but its unremarkable results and high risk make it an inadvisable form of treatment at this time [46].
Two separate trials of 100 men with PD showed promising results, including statistically significant improvements in the IIEF score and quality of life in patients receiving a one-month course of weekly ESWT therapy. While in the first trial (Palmieri et al [47], 2009), this effect was superior to the sham treatment, there was no statistically significant improvement in penile curvature or plaque size from baseline. In the second trial, ESWT therapy appeared to be augmented by a low daily dose of tadalafil, but again improvements in penile curvature and plaque size were not demonstrated [12]. A small RCT by Chitale et al [48] in 2010 showed no difference between patients receiving ESWT or sham therapy in any primary outcome measures (plaque size, curvature, IIEF).
Two small trials published in 2008 by Levine et al [49] and in 2009 by Gontero et al [50], respectively, showed only mild success with daily use of PTT over the course of six months. In the second trial, these results were not statistically significant, and found to be largely unsatisfactory to the patients involved. Penile remodeling has been found to be synergistic with collagenase treatment [10], suggesting there may be some role for PTT in combination with other treatment modalities. Further studies are needed to define the role of PTT in treating PD.
PD is characterized by disordered wound healing that is often associated with antecedent trauma to the erect penis. Yet not all individuals with a history of penile trauma go on to develop PD, supporting the idea that it is some interplay between trauma and genetic susceptibility that leads to the development of the disease. This idea is supported by the presence of myofibroblasts in PD lesions, which are cells that have long been implicated in the excessive collagen production that characterizes PD. These myofibroblasts originate from pluripotent stem cells in the tunica albuginea, but importantly, are not present in normal tunica albuginea tissue [51]. While therapies to date have focused on treating the consequences of the aberrant healing process caused by myofibroblasts, stem cell therapy offers the hope of altering the disease course before it even begins.
Even though stem cells are implicated in the pathogenesis of PD and offer hope for developing future treatments, barriers to implementing stem cell therapy in practice remain. An incomplete understanding of the pathophysiology of this complex disease has led to difficulty in reproducing the complexity of PD in an animal model. This lack of a universally accepted animal model for PD makes testing novel stem-cell therapies more difficult [52]. That being said, new therapies utilizing adipose tissue-derived stem cells (ADSCs) have been investigated in a rat model, and show early promise. Castiglione demonstrated in 27 male Sprague-Dawley rats that ADSC injection prevented fibrosis in the tunica albuginea during the active phase of a TGF-induced PD-like state [53]. The therapeutic action of these stem cells is thought to derive from their proangiogenic capacity that alters the cycle of vascular injury, ischemia, and fibrosis characteristic of the inflammatory phase of PD [52]. While still in its infancy, stem cell therapy offers perhaps the best hope for a definitive cure for PD.
Challenges in the treatment of PD are multifactorial. Reluctance by both patients and physicians to discuss this sensitive topic are a barrier to patients seeking treatment in the first place. Undoubtedly this contributes to the paucity of large RCTs that have been performed to date. The trials that do exist are often underpowered and utilize a plethora of different treatment modalities in a dizzying array of combinations. This multitude of treatment options for PD is a reflection of their relative ineffectiveness, and the lack of a nonsurgical 'gold standard' of treatment has made it difficult to compare the relatively few RCTs that do exist. Finally, the natural history of the disease in two distinct pathophysiologic phases (acute and chronic) further muddles the assessment of the treatment response, since treatments appropriate at one time may not be effective at another. This is compounded by a variable disease course that can progress, regress, or rarely, even resolve, without any treatment at all. Additional large-scale RCTs are needed in order to further elucidate the current best practices, and additional basic science research is needed to devise more effective treatments for this difficult condition.
Figures and Tables
Fig. 1
The deformities caused by Peyronie disease are many and varied. All patients appearing in this figure were intracavernosally injected with alprostadil to induce erection prior to photography. The pictures illustrate a dorsal bend with skin buckling (A), a pure lateral bend (B), a more gradual dorsal curvature (C), a dorsal curve with rotational deformity (as evidenced by the laterally displaced frenulum) (D), a circumferential plaque creating a 'waist' effect (E), and a ventral curve caused by a ventral plaque (F).
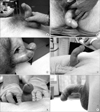
Table 1
Summary of recent studies investigating non-surgical treatment modalities for Peyronie disease

RCT: randomized controlled trial, ILI: intralesional injection, IIEF: International Index of Erectile Function, ED: erectile dysfunction, po: by mouth, bid: twice per day, tid: three times per day, PTT: penile traction therapy, ESWT: extracorporeal shock wave therapy, NS: normal saline, wk: week, mo: month.
References
1. Ralph D, Gonzalez-Cadavid N, Mirone V, Perovic S, Sohn M, Usta M, et al. The management of Peyronie's disease: evidence-based 2010 guidelines. J Sex Med. 2010; 7:2359–2374.

2. Gur S, Limin M, Hellstrom WJ. Current status and new developments in Peyronie's disease: medical, minimally invasive and surgical treatment options. Expert Opin Pharmacother. 2011; 12:931–944.

3. Schaeffer AJ, Burnett AL. Nonsurgical interventions for Peyronie disease: 2011 update. J Androl. 2012; 33:3–14.

4. Serefoglu EC, Hellstrom WJ. Treatment of Peyronie's disease: 2012 update. Curr Urol Rep. 2011; 12:444–452.

5. Gelbard M, Goldstein I, Hellstrom WJ, McMahon CG, Smith T, Tursi J, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013; 190:199–207.

6. Trost LW, Ates E, Powers M, Sikka S, Hellstrom WJ. Outcomes of intralesional interferon-α2B for the treatment of Peyronie disease. J Urol. 2013; 190:2194–2199.

7. Paulis G, Cavallini G, Brancato T, Alvaro R. Peironimev-Plus® in the treatment of chronic inflammation of tunica albuginea (Peyronie's disease). results of a controlled study. Inflamm Allergy Drug Targets. 2013; 12:61–67.

8. Mehrsai AR, Namdari F, Salavati A, Dehghani S, Allameh F, Pourmand G. Comparison of transdermal electromotive administration of verapamil and dexamethasone versus intra-lesional injection for Peyronie's disease. Andrology. 2013; 1:129–132.

9. Paulis G, Brancato T, D'Ascenzo R, De Giorgio G, Nupieri P, Orsolini G, et al. Efficacy of vitamin E in the conservative treatment of Peyronie's disease: legend or reality? A controlled study of 70 cases. Andrology. 2013; 1:120–128.

10. Gelbard M, Lipshultz LI, Tursi J, Smith T, Kaufman G, Levine LA. Phase 2b study of the clinical efficacy and safety of collagenase Clostridium histolyticum in patients with Peyronie disease. J Urol. 2012; 187:2268–2274.

11. Pavone C, Napoli G, Caruana G, Alonge V, Usala M, Abbadessa D. Safety and tolerability of local treatment with iloprost, a prostacyclin analogue, in patients with Peyronie's disease: a phase I study. BJU Int. 2012; 110:117–121.

12. Palmieri A, Imbimbo C, Creta M, Verze P, Fusco F, Mirone V. Tadalafil once daily and extracorporeal shock wave therapy in the management of patients with Peyronie's disease and erectile dysfunction: results from a prospective randomized trial. Int J Androl. 2012; 35:190–195.

13. Abern MR, Larsen S, Levine LA. Combination of penile traction, intralesional verapamil, and oral therapies for Peyronie's disease. J Sex Med. 2012; 9:288–295.

14. Chung E, Deyoung L, Brock GB. The role of PDE5 inhibitors in penile septal scar remodeling: assessment of clinical and radiological outcomes. J Sex Med. 2011; 8:1472–1477.

15. Smith JF, Shindel AW, Huang YC, Clavijo RI, Flechner L, Breyer BN, et al. Pentoxifylline treatment and penile calcifications in men with Peyronie's disease. Asian J Androl. 2011; 13:322–325.

16. Moskovic DJ, Alex B, Choi JM, Nelson CJ, Mulhall JP. Defining predictors of response to intralesional verapamil injection therapy for Peyronie's disease. BJU Int. 2011; 108:1485–1489.

17. Cavallini G, Biagiotti G, Lo Giudice C. Association between Peyronie disease and low serum testosterone levels: detection and therapeutic considerations. J Androl. 2012; 33:381–388.

18. Hashimoto K, Hisasue S, Kato R, Kobayashi K, Shimizu T, Tsukamoto T. Outcome analysis for conservative management of Peyronie's disease. Int J Urol. 2006; 13:244–247.

19. Safarinejad MR, Hosseini SY, Kolahi AA. Comparison of vitamin E and propionyl-L-carnitine, separately or in combination, in patients with early chronic Peyronie's disease: a double-blind, placebo controlled, randomized study. J Urol. 2007; 178:1398–1403.

21. Weidner W, Hauck EW, Schnitker J. Peyronie's Disease Study Group of Andrological Group of German Urologists. Potassium paraaminobenzoate (POTABA) in the treatment of Peyronie's disease: a prospective, placebo-controlled, randomized study. Eur Urol. 2005; 47:530–535.
22. Teloken C, Rhoden EL, Grazziotin TM, Ros CT, Sogari PR, Souto CA. Tamoxifen versus placebo in the treatment of Peyronie's disease. J Urol. 1999; 162:2003–2005.

23. Biagiotti G, Cavallini G. Acetyl-L-carnitine vs tamoxifen in the oral therapy of Peyronie's disease: a preliminary report. BJU Int. 2001; 88:63–67.

24. Safarinejad MR. Therapeutic effects of colchicine in the management of Peyronie's disease: a randomized double-blind, placebo-controlled study. Int J Impot Res. 2004; 16:238–243.

25. Prieto Castro RM, Leva Vallejo ME, Regueiro Lopez JC, Anglada Curado FJ, Alvarez Kindelan J, Requena Tapia MJ. Combined treatment with vitamin E and colchicine in the early stages of Peyronie's disease. BJU Int. 2003; 91:522–524.

26. Cavallini G, Biagiotti G, Koverech A, Vitali G. Oral propionyl-l-carnitine and intraplaque verapamil in the therapy of advanced and resistant Peyronie's disease. BJU Int. 2002; 89:895–900.

27. Safarinejad MR, Asgari MA, Hosseini SY, Dadkhah F. A double-blind placebo-controlled study of the efficacy and safety of pentoxifylline in early chronic Peyronie's disease. BJU Int. 2010; 106:240–248.

28. Levine LA, Latchamsetty KC. Treatment of erectile dysfunction in patients with Peyronie's disease using sildenafil citrate. Int J Impot Res. 2002; 14:478–482.

29. Safarinejad MR. Safety and efficacy of coenzyme Q10 supplementation in early chronic Peyronie's disease: a double-blind, placebo-controlled randomized study. Int J Impot Res. 2010; 22:298–309.

30. Safarinejad MR. Efficacy and safety of omega-3 for treatment of early-stage Peyronie's disease: aprospective, randomized, double-blind placebo-controlled study. J Sex Med. 2009; 6:1743–1754.
31. Levine LA, Merrick PF, Lee RC. Intralesional verapamil injection for the treatment of Peyronie's disease. J Urol. 1994; 151:1522–1524.

32. Rehman J, Benet A, Melman A. Use of intralesional verapamil to dissolve Peyronie's disease plaque: a long-term single-blind study. Urology. 1998; 51:620–626.

33. Bennett NE, Guhring P, Mulhall JP. Intralesional verapamil prevents the progression of Peyronie's disease. Urology. 2007; 69:1181–1184.

34. Shirazi M, Haghpanah AR, Badiee M, Afrasiabi MA, Haghpanah S. Effect of intralesional verapamil for treatment of Peyronie's disease: a randomized single-blind, placebo-controlled study. Int Urol Nephrol. 2009; 41:467–471.

35. Soh J, Kawauchi A, Kanemitsu N, Naya Y, Ochiai A, Naitoh Y, et al. Nicardipine vs. saline injection as treatment for Peyronie's disease: a prospective, randomized, single-blind trial. J Sex Med. 2010; 7:3743–3749.

36. Inal T, Tokatli Z, Akand M, Ozdiler E, Yaman O. Effect of intralesional interferon-alpha 2b combined with oral vitamin E for treatment of early stage Peyronie's disease: a randomized and prospective study. Urology. 2006; 67:1038–1042.

37. Hellstrom WJ, Kendirci M, Matern R, Cockerham Y, Myers L, Sikka SC, et al. Single-blind, multicenter, placebo controlled, parallel study to assess the safety and efficacy of intralesional interferon alpha-2B for minimally invasive treatment for Peyronie's disease. J Urol. 2006; 176:394–398.
38. Jordan GH. The use of intralesional clostridial collagenase injection therapy for Peyronie's disease: a prospective, single-center, non-placebo-controlled study. J Sex Med. 2008; 5:180–187.
39. Martin DJ, Badwan K, Parker M, Mulhall JP. Transdermal application of verapamil gel to the penile shaft fails to infiltrate the tunica albuginea. J Urol. 2002; 168:2483–2485.

40. Fitch WP 3rd, Easterling WJ, Talbert RL, Bordovsky MJ, Mosier M. Topical verapamil HCl, topical trifluoperazine, and topical magnesium sulfate for the treatment of Peyronie's disease--a placebo-controlled pilot study. J Sex Med. 2007; 4:477–484.
41. Riedl CR, Plas E, Engelhardt P, Daha K, Pflüger H. Iontophoresis for treatment of Peyronie's disease. J Urol. 2000; 163:95–99.

42. Montorsi F, Salonia A, Guazzoni G, Barbieri L, Colombo R, Brausi M, et al. Transdermal electromotive multi-drug administration for Peyronie's disease: preliminary results. J Androl. 2000; 21:85–90.
43. Levine LA, Estrada CR, Shou W, Cole A. Tunica albuginea tissue analysis after electromotive drug administration. J Urol. 2003; 169:1775–1778.

44. Di Stasi SM, Giannantoni A, Stephen RL, Capelli G, Giurioli A, Jannini EA, et al. A prospective, randomized study using transdermal electromotive administration of verapamil and dexamethasone for Peyronie's disease. J Urol. 2004; 171:1605–1608.

45. Greenfield JM, Shah SJ, Levine LA. Verapamil versus saline in electromotive drug administration for Peyronie's disease: a double-blind, placebo controlled trial. J Urol. 2007; 177:972–975.

46. Mulhall JP, Hall M, Broderick GA, Incrocci L. Radiation therapy in Peyronie's disease. J Sex Med. 2012; 9:1435–1441.

47. Palmieri A, Imbimbo C, Longo N, Fusco F, Verze P, Mangiapia F, et al. A first prospective, randomized, double-blind, placebo-controlled clinical trial evaluating extracorporeal shock wave therapy for the treatment of Peyronie's disease. Eur Urol. 2009; 56:363–369.

48. Chitale S, Morsey M, Swift L, Sethia K. Limited shock wave therapy vs sham treatment in men with Peyronie's disease: results of a prospective randomized controlled double-blind trial. BJU Int. 2010; 106:1352–1356.

49. Levine LA, Newell M, Taylor FL. Penile traction therapy for treatment of Peyronie's disease: a single-center pilot study. J Sex Med. 2008; 5:1468–1473.

50. Gontero P, Di Marco M, Giubilei G, Bartoletti R, Pappagallo G, Tizzani A, et al. Use of penile extender device in the treatment of penile curvature as a result of Peyronie's disease. Results of a phase II prospective study. J Sex Med. 2009; 6:558–566.
51. Gonzalez-Cadavid NF, Rajfer J. Treatment of Peyronie's disease with PDE5 inhibitors: an antifibrotic strategy. Nat Rev Urol. 2010; 7:215–221.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download