Abstract
Undifferentiated embryonal sarcoma of the liver (UESL) is a rare but aggressive primary tumor of the liver occurring most frequently in childhood. Its frequency in the adult population is extremely low. A 41-year-old Korean woman, who had previously undergone treatment for fallopian tube serous papillary adenocarcinoma, presented with a growing solitary mass in the right liver lobe after treatment. Although CA-125 level was normal, the mass was initially presumed to be metastasis from the primary fallopian tube carcinoma. On further examination, it was shown to be a UESL. Primary liver tumors should be considered in differential diagnoses in patients with fallopian tube cancer who subsequently raised liver tumors. This is particularly important when there is no direct evidence of recurrence of fallopian tube cancer.
Fallopian fube carcinoma (FTC) is a rare malignancy, comprising about 1% of all female genital tract cancer [1]. Its histologic appearance and clinical behavior resemble that of primary ovarian carcinoma. FTC usually manifests as widespread intraperitoneal metastasis [2]. A few patients are affected by an aggressive disease including liver, lung, or brain metastases [3]. An autopsy study of 428 patients with tubal cancer reported that over 40% of the patients with tubal cancer had evidence of liver metastases at the time of death [4]. Undifferentiated embryonal sarcoma of the liver (UESL), first documented in 1978, is a rare and highly malignant hepatic neoplasm of mesenchymal origin and shows a divergent differentiation [5,6]. UESL is a rare type of tumor and represents only 0.2% of all primary liver tumors [7]. Although UESL is considered a relatively major entity in pediatric liver malignancies, its frequency in the adult population is extremely low. In fact, few reports have focused on the general features of adult cases [8]. Furthermore, the detailed pathological characteristics of adult cases based on particular immuno-histochemistry are not yet clear. To our knowledge, no study has described the systemic pathology features of UESL in this journal. Our patient had received paclitaxel and cisplatin as adjuvant chemotherapy after resection of tubal cancer. Although CA-125 level was normal, Continuous follow-up scans found liver mass. We suspected persistant tubal cancer and performed liver resection. The results of the biopsy turned out to be primary UESL. We also review literature, diagnosis and treatment of UESL.
A 41-year-old Korean woman visited our hospital diagnosed with persistant tubal cancer. Although CA-125 level was normal, she had a metastatic hepatic nodule detected by computer tomography (CT) scans and positron-emission tomography-computed tomography (PET-CT) scans. She had visited local medical hospital two years ago with elevated serum CA-125 level and right adnexal mass on the CT scans. She had no experience in delivery and had a history of left salpingo-oophorectomy and myomectomy ten years and four years ago. Due to tubal pregnancy, The left salpingo-oophorectomy was performed. She had no history of underlying disease and specific family history. At local medical hospital, an exploratory laparotomy was performed. She was diagnosed with a tubal serous adenocarcinoma. Total abdominal hysterectomy, right salpingo-oophorectomy, and total omentectomy were done. Due to tumor invasion, splenectomy and distal pancreatectomy were performed. Final staging revealed IIIc fallopian tube carcinoma. Chemotherapy with cisplatin in combination with decetaxel was started 3 weeks after the operation. She had 9 cycles of first-line chemotherapy after the surgery at that hospital. After two months of first-line chemotherapy, CA-125 level slightly was elevated. We rechecked abdominal-pelvic CT scans and PET-CT scans. These scans showed a newly developed metastatic nodule in hepatic dome and seeding nodules in perihepatic space. New mass was found after two months. She was diagnosed with persistant of tubal cancer and treated with 2nd line chemotherapy with cisplatin in combination with paclitaxel. Because chemotherapy was performed at another hospital, we do not know why paclitaxel used as second chemotherapy. Despite the persistant state, they thought doxcltaxel was effective. After 3 cycles treatments of 2nd line chemotherapy, PET-CT scans revealed a nearly complete regression of persistant mass. The serum CA-125 level was within the normal range. After that she wanted to be transferred to our hospital.
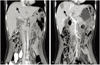
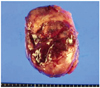
She visited our hospital with normal serum CA-125 level and a nearly complete regression of persistant hepatic nodule mass. She was 6 times more chemotherapy at our hospital. But PET-CT scans and CT scans after chemotherapy revealed an aggravating metastatic liver mass in hepatic dome area (Fig. 1). The serum CA-125 level was still within in normal range. She was consulted to hepatobiliary department. She was performed liver segmentectomy and was dignosed UESL (Fig. 2). MAID (doxorubicin/ifosfamide/dacarbazine) that she had were based on the experience derived from the treatment of sarcoma. Radiotherapy has been utilized with UESL, especially when surgical margins were not tumor free. Our patient received a successful surgery and was treated with chemoradiation therapy. In presence, She has been followed up with our departments.
UESL has a predilection for children and young adults of 20 years than younger. To our knowledge, few cases have been reported in patients over the age of 40 years [8]. The overall clinical outcome is poor with a long term disease-free survival rate of less than 30% in all series. UESL has no specific clinical features. It has been shown that children patients usually have the clinical symptoms of a large palpable mass with or without abdominal pain [9]. Most patients do not complain of nonspecific gastro-intestinal symptoms and signs, such as weight loss, nausea or anorexia, vomiting, jaundice, diarrhea, and fever [10]. Hepatomegaly may be present with large tumors, and liver function tests may be deranged, although frank jaundice is rare [5,11]. The nonspecific nature of the presenting symptoms makes clinical diagnosis extremely difficult without imaging or biopsy. UESL is not related to hepatitis and liver cirrhosis, and the liver function and tumor markers such as alpha fetoprotein (AFP), carcinoembryonic antigen (CEA) and CA 19-9 are normal in most cases. In our cases, laboratory tests showed mildly elevated levels of alanine aminotransferase and aspartate aminotransferase.
According to literature, the principal pathological features of UESL in children include an expansive intrahepatic growth with massive necrosis, hemorrhage, and occasional gelatinous appearance [5,6]. Macroscopically, UESL is usually a large, solitary and well-circumscribed mass with variable areas of hemorrhage, necrosis and cystic degeneration. Microscopically, it is composed of loosely arranged, medium-large spindles, oval and stellate pleomorphic cells with poorly defined cell borders, and giant cells with severe atypia. Although it's pathological origin remains unclear, ultrastructural and immune histochemical studies have shown its fibroblastic, histiocytic, lipoblastic, myoblastic, myofibroblastic, rhabdomyoblastic and leiomyoblastic differentiation [12]. Most UESL are diffusely positive for vimentin, a1-AT, and focally positive for cytokeratin, desmin, α-SMA, muscle-specific actin, CD68, myoglobin, non-specific enolase (Fig. 3), S100, and CD34, suggesting that embryonal sarcoma is an undifferentiated sarcoma, since it may display partial differentiation [12,13]. Although the lesion can be identified on ultrasonography and CT scan, contrast-enhanced magnetic resonance imaging (MRI) scan is the best imaging modality for characterization of UESL. Radiologic characteristics of UESL are enhanced peripheral rim, some solid portions at the periphery or adjacent to the septa, and discrepancy in internal architecture between CT and ultrasound scan [8]. But in recent years contrast-enhanced MRI scan is the best imaging. UESL shows hyperintense signal on T2 weighted MR images with low signal intensity septations. The majority of UESL is located in the right lobe, but it can also arise in the left lobe or in the bilateral lobes simultaneously. UESL in adults should be differentially diagnosed from carcinosarcoma, sarcomatoid or spindle-cell carcinoma, mesenchymal hamartoma, mixed hepatoblastoma with spindle-cell features, angiomyolipoma, and various other sarcomas (such as malignant fibrous histiocytoma, leiomyosarcoma, osteosarcoma, angiosarcoma, liposarcoma, melanoma, rhabdomyosarcoma or malignant schwannoma) [12]. Besides its large size, no other specific features can be used indifferential diagnosis of UESL from other hepatic masses. However, the morphology and complete immunohistochemical profiles of other hepatic masses are different from those of UESL. The prognosis of UESL is poor. Even after complete resection of the tumor, few UESL patients can achieve a long-term, disease-free survival. Since 1990, long-term survivors after multiagent chemotherapy have been reported and their outcome appears to have improved substantially over the last decades [14]. The chance of cure depends on radical resection and vigorous multiple approaches including chemotherapy [14]. To our knowledge, the current study is the first to demonstrate a significantly improved survival for patients with UESL who received adjuvant chemotherapy after undergoing a complete tumor resection compared with patients who underwent radical tumor resection alone [15]. The use of this treatment strategy was supported by more recent reports on UESL. Although radical surgery remains the mainstay of treatment, recent studies have shown improved survival with radical surgery in addition to the use of ifosfamide-based multiagent chemotherapy Thus, in most cases, the accepted standard treatment would consist of aggressive surgical resection and combination with chemotherapy either in neoadjuvant or adjuvant setting [7]. In this patient UESL masqueraded as a metastatic FTC. This case illustrates that the possibility of a primary liver tumor should be considered in patients with FTC who subsequently present with liver tumors, particularly when there is no direct evidence of recurrence of FTC. When we followed up patients with tubal and ovarian cancer, if liver mass does not correspond with CA-125 level, we must doubt primary liver tumor. Treatment should begin as soon as possible.
Figures and Tables
Fig. 1
(A) Newly noted metastatic nodule in the right hepatic dome (2010 November 8). (B) Aggravating process of the metastatic mass in the right hepatic lobe (2011 May 10).
References
1. Ou YC, Huang HY, Huang CC, Changchien CC, Tseng CW, Lin H. Primary fallopian tube carcinoma: clinicopathological analysis of 12 cases. Taiwan J Obstet Gynecol. 2011. 50:141–144.
2. Chobanian N, Dietrich CS 3rd. Ovarian cancer. Surg Clin North Am. 2008. 88:285–299.
3. Geisler JP, Geisler HE. Brain metastases in epithelial ovarian carcinoma. Gynecol Oncol. 1995. 57:246–249.
4. Rose PG, Piver MS, Tsukada Y, Lau TS. Metastatic patterns in histologic variants of ovarian cancer. An autopsy study. Cancer. 1989. 64:1508–1513.
5. Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978. 42:336–348.
6. Lack EE, Schloo BL, Azumi N, Travis WD, Grier HE, Kozakewich HP. Undifferentiated (embryonal) sarcoma of the liver. Clinical and pathologic study of 16 cases with emphasis on immunohistochemical features. Am J Surg Pathol. 1991. 15:1–16.
7. Kullar P, Stonard C, Jamieson N, Huguet E, Praseedom R, Jah A. Primary hepatic embryonal sarcoma masquerading as metastatic ovarian cancer. World J Surg Oncol. 2009. 7:55.
8. Psatha EA, Semelka RC, Fordham L, Firat Z, Woosley JT. Undifferentiated (embryonal) sarcoma of the liver (USL): MRI findings including dynamic gadolinium enhancement. Magn Reson Imaging. 2004. 22:897–900.
9. Baron PW, Majlessipour F, Bedros AA, Zuppan CW, Ben-Youssef R, Yanni G, et al. Undifferentiated embryonal sarcoma of the liver successfully treated with chemotherapy and liver resection. J Gastrointest Surg. 2007. 11:73–75.
10. Pachera S, Nishio H, Takahashi Y, Yokoyama Y, Oda K, Ebata T, et al. Undifferentiated embryonal sarcoma of the liver: case report and literature survey. J Hepatobiliary Pancreat Surg. 2008. 15:536–544.
11. Weitz J, Klimstra DS, Cymes K, Jarnagin WR, D'Angelica M, La Quaglia MP, et al. Management of primary liver sarcomas. Cancer. 2007. 109:1391–1396.
12. Zheng JM, Tao X, Xu AM, Chen XF, Wu MC, Zhang SH. Primary and recurrent embryonal sarcoma of the liver: clinicopathological and immunohistochemical analysis. Histopathology. 2007. 51:195–203.
13. Kiani B, Ferrell LD, Qualman S, Frankel WL. Immunohistochemical analysis of embryonal sarcoma of the liver. Appl Immunohistochem Mol Morphol. 2006. 14:193–197.
14. Walker NI, Horn MJ, Strong RW, Lynch SV, Cohen J, Ong TH, et al. Undifferentiated (embryonal) sarcoma of the liver. Pathologic findings and long-term survival after complete surgical resection. Cancer. 1992. 69:52–59.
15. Kim DY, Kim KH, Jung SE, Lee SC, Park KW, Kim WK. Undifferentiated (embryonal) sarcoma of the liver: combination treatment by surgery and chemotherapy. J Pediatr Surg. 2002. 37:1419–1423.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download