Abstract
Type A intestinal neuronal dysplasia is a congenital abnormality that is a very rare disease. Here, we report on a patient who had intestinal dilatation with serial changes and polydactyly, as shown on prenatal ultrasound. Bowel obstruction symptoms were shown at 16 days of life. An open abdominal surgery was performed. Malrotation of the bowel and narrowing of the area from 15 cm above the ileocecal valve were noted. Therefore, a right hemicolectomy, including lesions was performed. The patient was diagnosed with type A intestinal neuronal dysplasia by pathology.
Intestinal neuronal dysplasia (IND) is a congenital abnormality in the intestinal innervation system - hyperganglionosis [1]. IND is classified histologically as types A or B. Type A of IND is a congenital hypogenesis or agenesis of the innervation of the intestinal adrenergic sympathetic nerve and IND type B has abnormal parasympathetic innervation [2]. Of the patients with intestinal neuronal dysplasia, 5%-15% are type A and 70%-95% are type B [2]. Type A is very rare, and thus the precise incidence is not known. The incidence of type B is 1 in 4,000-60,000 live births [3]. IND is a congenital disease, the symptoms of which manifest during the neonatal period. Nevertheless, the prenatal features and diagnosis of intestinal neuronal dysplasia have not been reported.
We report a patient who had intestinal dilatation with serial changes and polydactyly, which was shown on prenatal ultrasound. The patient was diagnosed with type A intestinal neuronal dysplasia.
A 28-year-old primigravida was transferred to our hospital for evaluation of fetal polydactyly at 32-week gestation. At the time of ultrasonography, the fetus was in the breech presentation, and had polydactyly with 6 fingers on both hands and 6 toes on both feet. Within the abdomen, several dilated loops of bowel (approximately 1.72 cm) were noted (Fig. 1A, 1B). The volume of amniotic fluid was normal. Micromelia was shown in all long bones (<3rd percentile). After 2 weeks (34 weeks gestation), follow-up ultrasonography was performed. The diameters of bowel loops were shown to be decresed, but the number was increased (Fig. 1C). After 2 weeks (36 weeks gestation), follow-up ultrasonography was performed again. Several bowel loops disappeared, with only 3 small bowel loops remaining (Fig. 1D). At 39 weeks and 5 days, a female infant weighing 3,490 g was delivered by Cesarean section because of the breech presentation. The Apgar scores were 6 and 8 at 1 and 5 minutes, respectively; the condition of the neonate was good. Height was 50.5 cm, the head circumference was 38.5 cm, and the chest circumference was 31 cm. With the exception of polydactyly and micromelia, no additional external deformities were noted (Fig. 2). On abdominal X-ray obtained after birth, only mild dilatation of the intestine was observed. On abdominal ultrasound, the mild thickening of the intestinal wall was observed, but other abnormal features were not detected. From 4 days after birth, projectile vomiting symptoms were shown once a day, nonetheless, defecation patterns and breast feeding conditions were good. Thus, the patient was discharged at 9 days of life. At 16 days of life, the infant's weight had decreased to 3.17 kg. An upper gastrointestinal (UGI) series was obtained to evaluate green bilious vomit; the bowel movements were severely reduced, the jejunum was dilated, the ileum and the ascending colon were not observed, and the sigmoid colon was detected 24 hours after the UGI series. Because malrotation of the ileum and partial obstruction of the distal jejunum were suspected, open abdominal surgery was performed. At surgery, malrotation of the bowel and narrowing of the area from 15 cm above the ileocecal valve were noted, thus a right hemicolectomy, including the lesions, was performed with an end-to-end anastomosis of the ileum and colon. The length of the entire resected intestine was 24.0 cm; an area narrowed by approximately 6.0 cm was observed. Dilation of the bowel (13.0 cm in length) forming the transitional zone to the proximal area was observed. On microscopic examination, acute and chronic inflammatory findings were observed throughout the entire intestine, and findings consistent with enterocolitis and the destruction of crypts were observed (Fig. 3A). In addition, in the narrowed area as well as the dilated area, a hypertrophic and tortuous myenteric plexus with 2-5 cells per ganglia was observed (Fig. 3B), and it was proven clearly by immunohistochemical staining for the S-100 protein (Fig. 3C). Neither ectopic nor giant ganglia were detected. Because fresh tissues were not available, enzyme histochemistry for acetylcholinesterase was not performed. Based on the findings, the patient was diagnosed with type A intestinal neuronal dysplasia. Starting at day 4 after the operation, oral feeding was started, but projectile vomiting symptoms were shown. On abdominal X-ray obtained, significant dilatation of the intestine was observed. Total parenteral nutrition and oral feeding try was performed, but the patient condition had not improved. The patient died at post-operative 138 days due to multi-organ failure.
Type A of intestinal neuronal dysplasia is congenital abnormality and is a very rare disease. The ratio of male children-to-female children is 5:3 and it has a tendency to develop more often in male children. The time-to-diagnosis in male and female children is 5 and 3 months after birth and is diagnosed earlier in female children [4]. The clinical symptoms of type B manifest at more than 6 months after birth, and include progressive severe constipation. Many cases of type B are clinically indistinguishable from Hirschsprung's disease (aganglionosis). Type B is commonly associated with Hirschsprung's disease [5]. Barium enema can help with the differential diagnosis [6]. Clinical symptoms of type A is a functional ileus with hematochezia, with symptoms that progress rapidly. Thus, prompt surgical treatment is needed [7]. Without timely surgical intervention, the condition may deteriorate rapidly and lead to death [8]. The clinicopathologic findings in IND types A & B are shown in the Table 1, as compared to our case. From the above comparison, it can be concluded that our case fits in with type A.
Associated anomalies have been reported in 30.5% of patients (29 of 95 patients) with type B intestinal neuronal dysplasia; the most common anomaly is the gastrointestinal system, accounting for 67.4% of all anomalies, short stature was reported in the skeletal system [9]. Combined anomalies of IND type A reported infrequently because of the rarity. Congenital anomalies with colonic obstruction have been reported in patients with IND type A [10]. Congenital anomalies with colonic obstruction have been reported in patients with IND type A [10]. It has been reported that type A intestinal neuronal dysplasia may be associated with colitis or necrotic colitis based on histologic evaluation [7,11,12]. In addition to intestinal malrotation, our patient had musculoskeletal anomalies (micromelia and polydactyly).
Because clinical features of intestinal neuronal dysplasia are nonspecific and it is a very rare disease, the diagnosis may be delayed. All of the reported cases of intestinal neuronal dysplasia were diagnosed after birth on the basis of symptoms, even though intestinal neuronal dysplasia is a congenital disease. Prenatal features of intestinal neuronal dysplasia have not been reported. In our case, at 32 weeks gestation, long, dilated loops of bowel were demonstrated that changed with time. The finding of several dilated loops of bowel may exist in patients with small bowel obstruction, the most frequent cause of which is intestinal atresia [13]. The fetal sonographic findings of small bowel obstruction detected during pregnancy are very diverse, and are detected in the third trimester; moreover, the patterns are not consistent from patient-to-patient and can change with time. The ultrasonographic diagnosis of type A intestinal neuronal dysplasia may also show diverse features like other small bowel obstruction. If several bowel loops that change with time are shown during pregnancy, intestinal neuronal dysplasia must be considered in the differential diagnosis, and may be associated with deformities of the skeletal system. Thus, a comprehensive evaluation of the skeletal system is required.
Figures and Tables
Fig. 1
(A, B) Dilated multiple bowel loops (approximately 1.72 cm) at 32 weeks gestation. (C) Shortened and multiple bowel loops detected at 34 weeks gestation. (D) Normal sized bowel lumen at 36 weeks gestation.
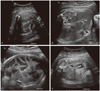
Fig. 3
(A) The mucosa showed a ruptured crypt with acute and chronic inflammation-arrow (H&E, ×40). (B) The colonic wall showed hypertrophic and tortuous myenteric plexus-arrow (H&E, ×100). (C) Immunohistochemistry for S-100 protein showed strong positivity for hypertrophic nerve bundles-arrow (×100).

References
1. Meier-Ruge W. Casuistic of colon disorder with symptoms of Hirschsprung's disease (author's transl). Verh Dtsch Ges Pathol. 1971. 55:506–510.
2. Fadda B, Maier WA, Meier-Ruge W, Schärli A, Daum R. Neuronal intestinal dysplasia. Critical 10-years' analysis of clinical and biopsy diagnosis. Z Kinderchir. 1983. 38:305–311.
3. Martucciello G, Caffarena PE, Lerone M, Mattioli G, Barabino A, Bisio G, et al. Neuronal intestinal dysplasia: clinical experience in Italian patients. Eur J Pediatr Surg. 1994. 4:287–292.
4. Meier-Ruge W. Epidemiology of congenital innervation defects of the distal colon. Virchows Arch A Pathol Anat Histopathol. 1992. 420:171–177.
5. Kobayashi H, Hirakawa H, Surana R, O'Briain DS, Puri P. Intestinal neuronal dysplasia is a possible cause of persistent bowel symptoms after pull-through operation for Hirschsprung's disease. J Pediatr Surg. 1995. 30:253–257.
6. Montedonico S, Acevedo S, Fadda B. Clinical aspects of intestinal neuronal dysplasia. J Pediatr Surg. 2002. 37:1772–1774.
7. Schärli AF, Meier-Ruge W. Localized and disseminated forms of neuronal intestinal dysplasia mimicking Hirschsprung's disease. J Pediatr Surg. 1981. 16:164–170.
8. Rajalakshmi T, Makhija P, Babu MK, Kini U. Intestinal neuronal dysplasia type A. Indian J Pediatr. 2003. 70:839–841.
9. Corduk N, Koltuksuz U, Bir F, Karabul M, Herek O, Sarioglu-Buke A. Association of rare intestinal malformations: colonic atresia and intestinal neuronal dysplasia. Adv Ther. 2007. 24:1254–1259.
10. Briner J, Oswald HW, Hirsig J, Lehner M. Neuronal intestinal dysplasia: clinical and histochemical findings and its association with Hirschsprung's disease. Z Kinderchir. 1986. 41:282–286.
11. Puri P. Variant Hirschsprung's disease? J Pediatr Surg. 1997. 32:149–157.
12. Schofield DE, Yunis EJ. What is intestinal neuronal dysplasia. Pathol Annu. 1992. 27:249–262.
13. Reyes HM, Meller JL, Loeff D. Neonatal intestinal obstruction. Clin Perinatol. 1989. 16:85–96.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download