Abstract
Chordomas originate from remnants of the embryonic notochord and account for < 2% of all malignant bone tumors. Chordomas have a high rate of local recurrence. However, spinal cerebrospinal fluid (CSF) seeding of a chordoma is extremely rare. Here, we present a very rare case of clival chordoma with spinal seeding. Radiologists should consider spinal CSF seeding of a clival chordoma, particularly when accompanied by signs of dural perforation or caudal extension.
Chordomas originate from remnants of the embryonic notochord and account for < 2% of all malignant bone tumors (1). Although chordomas are low-grade tumors, they have a high rate of local recurrence, which makes clinical manifestation of a chordoma similar to a malignant tumor (2). In most cases, the initial clinical manifestations of a chordoma are neurological deficits caused by direct local invasion or the mass effect on adjacent structures (3). The incidence of chordoma metastasis varies widely at 3-48%. Common sites for chordoma metastasis are the lungs, liver, bone, and lymph nodes without clear predominance (4). We experienced a case of spinal cerebrospinal fluid (CSF) seeding of a clival chordoma, which was an uncommon metastatic site.
An 18-year-old woman presented with hip pain for the past month. She had visited the emergency room 5 years ago for acute onset dysarthria. At that time, brain magnetic resonance imaging (MRI) was performed, and a brain tumor was detected incidentally. The brain MRI showed a lobulated heterogeneously enhancing mass, involving the entire clivus and extending to the upper cervical level with compression of the brain stem. The mass showed high signal intensity on sagittal T2-weighted images and was considered a clival chordoma (Fig. 1A-C). The patient underwent decompression of the mass through the trans-sphenoidal approach. The dura was focally perforated due to direct invasion by the tumor. The histopathological examination of the clival mass was consistent with chordoma (Fig. 1D). Less than 1% of the cells were Ki-67 positive. Decompression had been repeated three times since then and follow-up brain MRI was performed after 5 years.
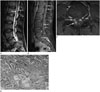
She presented with hip pain this time, and contrast-enhanced spinal MRI was performed for further evaluation. MRI of the entire spine revealed multiple small homogeneously enhancing lesions on the pial surfaces of the distal conus and in the lumbosacral subarachnoid space (Fig. 2A-C). We performed positron emission tomography-computed tomography examination, which showed a focal hypermetabolic lesion at the upper sacral level in the spinal canal. We suspected spinal CSF seeding of the tumor based on these findings.
The patient underwent an excisional biopsy for the intradural enhancing nodule at the L1 level to obtain a tissue specimen. The enhanced nodule was attached to the dorsal pial surface of the spinal cord and cauda equina. The mass was hard and tightly intermingled to nerves.
The histopathological features of the excised mass revealed an extensive myxoid stroma with physaliferous cells containing a very large vacuolated cytoplasm with prominent vesicular nuclei. Few mitotic figures were detected (Fig. 2D). These findings were consistent with a chordoma.
A chordoma is a rare tumor and most primary chordomas are located in the sacrococcygeal region (62-73%), with only 7-10% found in the clivus (4). A clival chordoma is relatively rare among intracranial tumors, with an incidence rate of 0.2-0.4% (5).
Metastasis of a chordoma is not well documented. The low incidence and slow growth of the disease make it difficult to diagnose (4). Common metastatic sites for a chordoma are the lungs, liver, bone, and lymph nodes, without clear predominance (4).
Spinal CSF seeding from a clival chordoma is extremely rare, and only nine cases have been reported since a case report by Holzner (6) in 1954. Including our case (case 10), the patient's were 6-59 years of age. The locations of the metastatic lesions were unknown in three cases, the thoracolumbar area in four cases, the cervicothoracic area in two cases, and the cauda equina in one case. Six cases occurred in men, three in women, and one unknown. The period until detection of spinal CSF seeding varied from the time of the diagnosis to 11 years (7).
The radiological features for spinal CSF seeding of a clival chordoma are not well-known, as only two cases have reported spinal CSF seeding of a clival chordoma. Krol et al. (8) reported CSF seeding of a clival chordoma on MR myelography as an irregular multi-nodular filling defect. The myelographic findings of spinal CSF seeding are non-specific, as many metastatic tumors share similar imaging features.
A surgical procedure is a predisposing factor for spreading a clival chordoma to the spinal intradural space. In particular, tumor spreading can occur frequently when surgical manipulation involves the dural space (9). The classic approach for a clival chordoma is from the extra-dura without destroying the intradural space but manipulation of the intradural space is inevitable, as some tumors have already invaded the intradural space. In our case, the dura had been perforated during the initial intraoperative inspection. Therefore, we assumed that the spinal CSF seeding may have been to the dural perforation due to direct invasion of the tumor.
The proliferative capability of a chordoma is indicated by the Ki-67 labeling index (LI). A higher Ki-67 LI is observed with a faster growth rate. In addition, the tumor Ki-67 LI tends to increase during long-term follow-up (10). In our case, the Ki-67 LI was low and we determined the Ki-67 LI only at the first biopsy specimen. However, if serial Ki-67 LIs were determined in our case, we may have suspected distant spinal CSF seeding of the chordoma due to the increase in the Ki-67 LI. We suggest that the Ki-67 should be calculated initially and at the follow-up. Further evaluation, such as spinal MRI, should be performed to rule out distant metastasis of the chordoma if the Ki-67 LI is at the follow-up.
Spinal CSF seeding of a clival chordoma is extremely rare, and a prolonged diagnosis of spinal CSF seeding of a clival chordoma is even more uncommon. In our case, we suspected that spinal CSF seeding of the chordoma was related to the dural perforation and caudal extension of the mass.
Although rare, spinal CSF seeding can occur, particularly when accompanied by dural perforation or caudal extension of the mass. Therefore, when a high Ki-67 LI, dural perforation, or caudal extension of the mass are detected in pateint with a clival chordoma, long-term follow-up and close observations with spinal MRI should be carried out to rule out the possibility of spinal CSF seeding from the clival chordoma.
Figures and Tables
Fig. 1
18-year-old woman with clival chordoma.
A-C. Sagittal brain MR images (T2-weighted, T1-weighted, fat-suppressed contrast-enhanced T1-weighted) shows high T2 signal intensity, contour-bulging heterogeneously enhancing mass (arrow), involving entire clivus, anterior perimedullary, lower prepontine cistern, with posterior compression to the brain stem.
D. Micrograph shows physaliferous (having bubbles or vacuoles) cells with extensive myxoid stroma. The cells (arrow) are very large, with vacuolated cytoplasm, prominent vesicular nucleus and rare mitotic figures (hematoxylin and eosin, × 400).
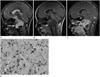
Fig. 2
18-year-old woman with clival chordoma.
A, B. Sagittal spine MR images (T2-weighted, fat-suppressed contrast-enhanced T1-weighted) show multifocal small nodular enhancing lesions (arrows) in the pial surfaces of distal conus, and lumbosacral spinal cord.
C. Axial fat-suppressed contrast-enhanced T1-weighted images show multiple enhancing nodules (arrows) in the pial surfaces of spinal cord.
D. On microscopic images, irregular clusters of neoplastic cells showing small bland nuclei and well-defined, homogeneous and eosinophilic cytoplasm are present in a mucoid matrix (hematoxylin and eosin, × 100). It is the typical histologic findings of chordoma.
References
1. Dahlin DC. Bone tumors: general aspects and data on 6221 cases. 3rd ed. Springfield: Charles C Thomas;1978. p. 329–343.
2. Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000; 88:2122–2134.
3. Wang CC, James AE Jr. Chordoma: brief review of the literature and report of a case with widespread metastases. Cancer. 1968; 22:162–167.
4. McPherson CM, Suki D, McCutcheon IE, Gokaslan ZL, Rhines LD, Mendel E. Metastatic disease from spinal chordoma: a 10-year experience. J Neurosurg Spine. 2006; 5:277–280.
5. Zulch KJ. Brain tumors. Their biology and pathology. 3rd ed. Berlin Heidelberg: New York: Tokyo: Springer-Verlag;1986. p. 476–479.
6. Holzner H. [Unusual metastasis of chordoma]. Zentralbl Allg Pathol. 1954; 92:12–18.
7. Asano S, Kawahara N, Kirino T. Intradural spinal seeding of a clival chordoma. Acta Neurochir (Wien). 2003; 145:599–603. discussion 603
8. Krol G, Sze G, Arbit E, Marcove R, Sundaresan N. Intradural metastases of chordoma. AJNR Am J Neuroradiol. 1989; 10:193–195.
9. Stough DR, Hartzog JT, Fisher RG. Unusual intradural spinal metastasis of a cranial chordoma. Case report. J Neurosurg. 1971; 34:560–562.
10. Holton JL, Steel T, Luxsuwong M, Crockard HA, Revesz T. Skull base chordomas: correlation of tumour doubling time with age, mitosis and Ki67 proliferation index. Neuropathol Appl Neurobiol. 2000; 26:497–503.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download