Abstract
We report a rare case of a direct communication-forming fistula between the left main pulmonary artery and left atrial appendage detected on computed tomography and color Doppler echocardiography.
Direct communication between the pulmonary artery and the left atrium (LA) is a rare congenital variation of arteriovenous fistula. Since its initial description by Friedlich et al. (1), approximately 60 cases have been reported in the literature and most of them involved communication from the right pulmonary artery to the LA (2). Most cases reported are of congenital origin, while some other cases appear to be of posttraumatic origin. A hemodynamic right-to-left (R-L) shunt may result in cyanosis, clubbing, dyspnea, polycythemia, and paradoxical systemic embolization (34). In this study, we report an anomalous case involving direct communication between the left main pulmonary artery (LMPA) and the left atrial appendage, which was diagnosed by computed tomography (CT) and color Doppler echocardiography.
A 23-year-old female patient was referred to our hospital with a cardiac murmur on physical examination. She had suffered, since childhood, from intermittent shortness of breath with physical exertion such as when participating in school sports day. She had no clinical history of trauma or surgery that could be related to any cardiac abnormality and also denied any history of smoking, diabetes mellitus, hypertension, or rheumatic fever. Upon physical examination, no cyanosis or clubbing of the fingers was noted. Her height (164 cm) and weight (52 kg) were within normal range, and her blood pressure was 132/80 mm Hg. Laboratory tests were also within normal range including hemoglobin level, hematocrit level, partial pressure of oxygen in arterial blood (PaO2), and saturation of oxygen in arterial blood (SaO2). Auscultation obtained in our hospital showed a grade 3 to 4 continuous murmur in the pulmonary valve area and a simple chest radiography revealed no abnormalities. Electrocardiography demonstrated a normal sinus rhythm with a heart rate of 67 bpm. Transthoracic echocardiography revealed mild thickening of the mitral valve and prolapse of the anterior mitral valve associated with mild mitral regurgitation in systole observed on echocardiography. The morphology and function of the pulmonary valve was normal; however, an abnormal backward flow was detected above the pulmonary valve in diastole on color Doppler (Fig. 1). Contrast-enhanced CT was carried out to evaluate the anatomy and to rule out patent ductus arteriosus. CT revealed an abnormal direct communication from the inferior aspect of the LMPA, close to the bifurcation site of the pulmonary trunk, to the left atrial appendage rather than patent ductus arteriosus (Fig. 2). The maximum diameter of the communicating fistula vessel was 6 mm. Dilatation of the pulmonary trunk, LMPA and right ventricle were also noted, but no associated hypertrophy of the right ventricular free wall was observed. The right pulmonary artery and pulmonary venous return were normal and no other shunt evidence associated with cardiac structure and no lung parenchymal anatomy abnormalities were detected. According to the cardiologist's discretion, selective pulmonary angiography, early intervention, and surgical correction were not conducted, and a regular follow-up schedule was made.
In the case described herein, a rare congenital anomaly of a direct communication-forming fistula between the LMPA and the left atrial appendage was detected on CT and color Doppler echocardiography.
Although there has been one previous case of direct communication between the left pulmonary artery and the LA, which was diagnosed via contrast-enhanced echocardiography and selective pulmonary angiography, our case involved diagnosis via contrast-enhanced CT and color Doppler echocardiography (5).
Clinical manifestations can appear from infancy to late adulthood; those who evidence late symptoms tend to exhibit a mild clinical course relative to patients who present in infancy (6).
In cases of direct communication between the pulmonary artery to LA, usually via an R-L shunt, symptoms including cyanosis of the lips, cheeks, and extremities, dyspnea on exertion and digital clubbing may arise, depending on the size and location of the fistula (7). Therefore, in cases in which cyanosis occurs but the structure of the heart is normal, direct communication between the pulmonary artery and LA might be suspected; laboratory tests may demonstrate arterial hypoxia and polycythemia. LA dilatation and left ventricular hypertrophy may be noted on electrocardiography. Contrast-enhanced echocardiography and selective pulmonary angiography directly demonstrate evidence of the shunt. Rapid opacification of the LA and poor opacification of the lung could be observed on selective pulmonary angiography. Upon cardiac catheterization, LA O2 saturation was markedly reduced and showed poor response to the extra O2 (7).
Early diagnosis is essentially based on hemodynamics and owes to the bypass of pulmonary filtering, which allows microorganisms to enter directly into the systemic circulation, possibly resulting in cerebral or systemic embolization or abscess. Other complications may include pulmonary edema associated with left heart insufficiency with irreversible pulmonary hypertension and consequent polycythemia.
According to previous reports, even though this anomaly results from an abnormal communication between the pulmonary artery and the pulmonary veins, which are subsequently incorporated into the LA, we remain unsure of the etiology of the direct communication with the fistula between the LMPA and the appendage of LA, as well as the difference in incidence between the right-sided and left-sided fistula (8).
In our case, there was an atrial-to-pulmonary backward flow (L-R shunt) in diastole that was clearly observed on color Doppler echocardiography. We thought that there might be another shunt flow of a pulmonary-to-atrial flow (R-L shunt) in systole, suggesting a bi-directional shunt flow.
For treatment of this condition, surgical ligation or division is recommended. Recently, interventional treatment using an Amplatzer ductal occluder has been used as an alternative, with relatively good results (910).
In summary, we present herein a rare case of direct communication-forming fistula between the LMPA and the left atrial appendage, as revealed by CT angiography and color Doppler echocardiography in a patient with cardiac murmur on physical examination. Further study will be required to establish the etiology, incidence, and clinical significance of left-sided pulmonary artery-originated fistula, in contradistinction to right-sided fistula.
Figures and Tables
Fig. 1
A 23-year-old woman with a continuous cardiac murmur. A transthoracic color Doppler echocardiography was performed on the pulmonary trunk in systole (A) and diastole (B). Note the dilatation and regurgitant flow (red color flow, arrow) in the pulmonary trunk in diastole on the parasternal view
Note.-PT = pulmonary trunk, LAA = left atrial appendage area

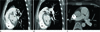
Fig. 2
A 23-year-old woman with a continuous cardiac murmur. Oblique maximum intensity projection (MIP) and axial images of computed tomography show an abnormal direct communication (arrow) from the inferior aspect of the LMPA, close to the bifurcation site of the pulmonary trunk, to the left atrial appendage (A-C). The maximum diameter of the communicating fistula vessel was 6 mm in size.
Note.-Ao = ascending aorta, LMPA = left main pulmonary artery, LA = left atrium, LV = left ventricle, RV = right ventricle
References
1. Friedlich A, Bing RJ, Blount SG Jr. Physiological studies in congenital heart disease; circulatory dynamics in the anomalies of venous return to the heart including pulmonary arteriovenous fistula. Bull Johns Hopkins Hosp. 1950; 86:20–57.
2. Mohanty SR, Yadav R, Kothari SS, Airan B. Right pulmonary artery left atrium communication. Ann Thorac Surg. 2000; 69:269–271.
3. Lucas RV Jr, Lund GW, Edwards JE. Direct communication of a pulmonary artery with the left atrium. An unusual variant of pulmonary arteriovenous fistula. Circulation. 1961; 24:1409–1414.
4. Orlick AE, Hultgren HN, Stoner JD, Barry WH, Wexler L, Dong EV Jr. Traumatic pulmonary artery--left atrial fistula: an unusual case of cyanosis in an adult. Am Heart J. 1979; 98:366–370.
5. Karnik AM, Nilsson U, Vijayaraghavan G, Hashmi J, Shuhaiber H. Direct communication between the left pulmonary artery and the left atrium. Chest. 1989; 96:937–939.
6. Faizal A, Madhu Sankar N, Murthy KS, Cherian KM. Right pulmonary artery-to-left atrial fistula. Asian Cardiovasc Thorac Ann. 2002; 10:80–82.
7. Liu L, Wei X, Pan T. Congenital right pulmonary artery-to-left atrial fistula. Asian Cardiovasc Thorac Ann. 2010; 18:373–375.
8. Ohara H, Ito K, Kohguchi N, Ohkawa Y, Akasaka T, Takarada M, et al. Direct communication between the right pulmonary artery and the left atrium. A case report and review of the literature. J Thorac Cardiovasc Surg. 1979; 77:742–747.
9. Francis E, Sivakumar K, Kumar RK. Transcatheter closure of fistula between the right pulmonary artery and left atrium using the Amplatzer duct occluder. Catheter Cardiovasc Interv. 2004; 63:83–86.
10. Batinica S, Gagro A, Bradić I, Marinović B. Congenital pulmonary arteriovenous fistula: a rare cause of cyanosis in childhood. Thorac Cardiovasc Surg. 1991; 39:105–106.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download