Abstract
Purpose
To describe how to make a swine hemodialysis fistula model and report our initial experience to test the feasibility of endovascular radiation therapy with Holmium-166 filled balloon catheters.
Materials and Methods
The surgical formation of arterio-venous fistula (AVF) was performed by end-to-side anastomosis of the bilateral jugular vein and carotid artery of 6 pigs. After 4 weeks, angiograms were taken and endovascular radiation was delivered to the venous side of AVF with Holmium-166 filled balloon catheters. Pigs were sacrificed 4 weeks after the radiation and AVFs were harvested for histological examination.
Results
All animals survived without any morbidity during the experimental periods. The formation of fistula on the sides of necks was successful in 11 of the 12 pigs (92%). One AVF failed from the small jugular vein. On angiograms, 4 of the 11 AVFs showed total occlusion or significant stenosis and therefore, endovascular radiation could not be performed. Of 7 eligible AVFs, five underwent successful endovascular radiation and two AVFs did not undergo radiation for the control. Upon histologic analysis, one non-radiated AVF showed total occlusion and others showed intimal thickening from the neointimal hyperplasia.
For patients undergoing hemodialysis, vascular access failure of the arteriovenous native fistula or graft is a vexing problem. Major causes for this include venous stenosis or occlusion due to neointimal hyperplasia. While much effort has been put to reduce the incidence of neointimal hyperplasia with various methods, none of them have been effective clinically (123). For hemodialysis related to venous stenosis, percutaneous endovascular treatment has become an accepted alternative to surgical reconstruction, with a high success rate and prolonged patency (4). Among other alternative treatments, endovascular radiation therapy has been shown to decrease neointimal hyperplasia in an animal model (5) and was applied to coronary or peripheral arteries following balloon angioplasty for the treatment of stenosis (67). However, a limited number of reports have been published concerning the effect of endovascular radiation therapy on venous stenotherasis of hemodialysis fistula.
Animal models, which are appropriate for various interventional treatments, have recently been developed. The conditions of animal models which are appropriate for the prevention of vascular access stenosis include the histopathologic changes of lesions which are similar to those of human subjects, as well as the prompt progression of stenosis. Besides, the reactions ensued following the damage to vascular access must be similar to those seen in human subjects (8). To date, animal studies pertaining to hemodialysis access have many things to determine, such as the kind of subject animal, optimal artery and vein for arterio-venous fistula (AVF), and the details of surgical techniques for creating an AVF (9). Furthermore, most of the animal studies were performed on arteriovenous grafts and its in vivo environment is different from AVFs which use native vessels.
The purposes of this study are to describe how to make a swine hemodialysis fistula model and to report our initial experience in testing the feasibility of endovascular radiation therapy with Holmium-166 filled balloon catheters.
Experimental animals used for this study were six pigs weighing approximately 30 kg, which were raised for about three months.
After inserting an endotracheal tube, experimental animals were anesthetized using xylazine, telazol, and atropine, and maintained using 1-3% isoflurane. In the first two experimental animals, the anastomosis between the iliac artery and vein was attempted. In these animals, however, it was confirmed that the iliac arteries and veins were not appropriate for the formation of an AVF because the location of the vessels were too deep and the artery and vein were remotely present. Consequently, we used the common carotid artery and internal jugular vein. Both sides of the common carotid arteries and internal jugular veins were exposed to a 8-10 cm longitudinal incision made anteriorly to the neck of pigs and vascular control was obtained with the use of coronary bulldog clamps following heparin administration. After making a 6 mm length longitudinal incision at the common carotid artery, the end of the dissected internal jugular vein was anastomosed to the side of the common carotid artery bilaterally, using a 6/0 polypropylene suture. The fascia and skin were sutured using a 3/0 Dexon silk. All the experimental animals were given aspirin at a daily dose of 325 mg persistently starting a few days before the surgery to prevent thrombus formation.
Four weeks following the formation of an AVF, an angiography on the AVF was performed to confirm the maturation of veins in experimental animals. Following the same systemic anesthesia used in the previous fistula formation, the femoral artery was surgically exposed and an 8-French (F) introducer sheath was inserted using the Seldinger method. The distal end of the introducer was placed in the aortic arch and a 5-F catheter progressed through the introducer sheath to the anastomosis site of the AVF. Following the infusion of angiographic contrast media, the angiography of AVF was performed to examine the shape of the fistula and the veins. Following this, the diameter of the veins was measured.
After finishing the angiography, endovascular irradiation was applied using Holmium-166 filled balloon catheter at the vein segment at 3 cm distal to the anastomosis, which is known as the most vulnerable site of venous stenosis for an AVF. The diameter and length of the balloon catheter measured 6 mm by 4 cm, respectively.
On the morning of the very experimental day, Holmium-166 was prepared by the dissolution of distilled water and Holmium-166 nitrate pentahydrate [166-Ho (NO3) 35H2O], which was generated after the activation of 165Hol nitrate pentahydrate [165-Ho (NO3) 35H2O] in a nuclear reactor at the Korea Atomic Research Institute (Daejeon, Korea). After wearing a lead glove, Holmium-distilled water solution was filled into a balloon catheter using an inflation device and then the endovascular irradiation was performed for all the AVFs created except two for the controls.
The target radiation dose was set at 20 Gy, which has been known to be the optimal dose of the internal radiation therapy for coronary arteries (1011). The radiation dosimetry was calculated by establishing the radiation source using a phantom and then directly irradiating it to the object. The object was made with thermoluminescence material and the radiation absorption dose was directly measured. To achieve this, a radiochromic film (Gafchromic MD55-2, ISP Technologies Inc., Wayne, NJ, USA) and videodensitometer (WP700, Well-höfer Co, Schwarzenbruck, Germany) were used and the calibration curve of optical density depending on the radiation dose was obtained. Using this relationship, after the radioactivity of Holmium in a balloon catheter with a diameter of 6 mm was obtained according to a radiation dose of 20 Gy, the following table was prepared (12) (Table 1). Then, the target radiation dose was irradiated to each experimental animal.
For example, to irradiate 20 Gy to a 0.5 mm depth from the surface of a balloon catheter, the time of irradiation with a balloon catheter filled with Holmium-166 solution with a radioactivity of 200 mCi/1 mL was 3.6 minutes.
Each animal was sacrificed at 4 weeks after the radiation. Prior to sacrificing each experimental animal, the cervical area was incised, thereby exposing the AVF. After sacrificing the experimental animals, the venous sides of the AVFs were resected and then fixed in a 10% formalin solution. The outer appearance of the veins was grossly examined based on a gross photograph and then embedded in a paraffin block. These paraffin-embedded blocks were sectioned and then prepared into tissue sample. Following the hematoxylin-eosin staining, light microscopy was performed for a histopathologic analysis.
The surgical formation of AVF was successful in 11 of 12 sides of the necks of experimental animals (92%) (Fig. 1), and a thrill was palpable at the venous limb of the AVFs immediately after the surgery. One case of surgery failed because the diameter of the vein was too small (2 mm) to be sutured with the arterial wall. All the animals survived during the entire experimental period without any morbidity.
On angiography taken 4 weeks after the formation of the AVF, total occlusion was observed in 2 of 11 veins. In 2 other veins, significant stenosis (stenosis greater than 50% of diameter) was identified (Fig. 2) and a balloon catheter could not be passed through the stenosis. Therefore, internal radiation could not be performed as scheduled for those 4 AVFs. The causative factors for the occlusion and the significant stenosis were unknown. The seven remaining AVFs ensured a sufficient lumen of the venous limb to advance a balloon catheter. However, they also showed irregular strictures of the venous limbs with non-uniform diameters from 3 mm to 8 mm and all the strictures were located at the juxta-anastomosis segment of the venous limb about 10 cm within the anastomosis site. Consecutive endovascular radiation was successfully given on venous limbs of 5 of 7 patent AVFs, and 2 AVF was spared for the control. On histologic examination taken 4 weeks later, 1 of 2 non-radiated control AVFs was totally occluded and the light microscopic findings depicted obliteration of the venous lumen by neointimal hyperplasia, which consisted of the proliferation of the smooth muscle cells, extracellular matrix, and the formation of the microvessels. A histologic analysis of other AVFs also showed neointimal hyperplasia consisting of similar microscopic structures to the obstructed AVF (Fig. 3).
Mean patency rate of AVFs for hemodialysis in patients with chronic renal failure is known to be about 2.9 years and, repeated surgery or interventions are required after then because of the venous stenosis or occlusion by neointimal hyperplasia (13). Many studies have been conducted to examine the pathology and pathogenesis of neointimal hyperplasia. Major causes of the neointimal hyperplasia in AVFs include the damage of venous wall due to the frequent puncture of blood vessels and the high pressure environment due to the arterio-venous anastomosis. The venous puncture triggers the damage of the intima and releases mitogen, which in turn promotes the migration of smooth muscle cells into the blood vessel and inducing the activation of platelets and the adhesion to the vascular wall (14). Nevertheless, few methods have been reported to be effective for the prevention of neointimal hyperplasia.
To date, many attempts have been made to prevent the occurrence of vascular access stenosis, mainly by the systemic rather than local treatment. However, this caused many systemic adverse effects without significant treatment effects. Local treatment modalities which have been made, include the application of polymer binding antiproliferative drugs such as paclitaxel and dipyridamole, to the site of vascular access, as well as the local use of irradiation and the placement of drug-eluting stents (15). In recent years, ongoing studies have been conducted to examine gene therapy.
Of these studies, the endovascular radiotherapy has been examined actively to prevent the neointimal hyperplasia in the relevant clinical areas dealing with the heart, peripheral artery, and AVF (16). The known mechanisms are the suppression of cellular proliferation, the inactivation of cells, the decreased synthesis of extracellular matrix, and the inhibition of cellular migration. According to the study about endovascular radiotherapy using Ir-192 in cardiac artery (Scripps Coronary Radiation to Inhibit Proliferation Post Stenting; SCRIPPS), the neointimal hyperplasia was decreased in approximately 70% of total cases (17). A Beta Energy Restenosis Trial (BERT) reported that the neointimal hyperplasia was decreased in more than 50% of patients who underwent treatment using Sr-90 for the cardiac artery (18). Most of the studies about endovascular radiation therapy have been focusing on arterial systems, and not much on the venous systems or hemodialysis fistulas.
Most preclinical studies of methods or devices which may prolong access patency have used a canine or swine model for AVF. The response of canine veins to the presence of an AVF was predictive of a similar response in man, because that model inconsistently led to neointimal hyperplasia at a slower rate than in a man (8). Pigs have been used in cardiac research for decades. The aggregate experience of many studies has led to the acceptance of the porcine model as the non-primate model where the results of therapies directed at vascular stenosis are the most predictive of similar results in the human arterial system (8). However, limited numbers of studies have been conducted and no appropriate methods dealing with the pig model have been established to examine the treatment effects on the neointimal hyperplasia of AVF (19). Our protocol included methods of the formation of the fistula, performing angiography, and endovascular radiation, which could be helpful to forthcoming studies. We think four week intervals between the formation of the AVF and radiation treatment are needed for further modifications to make uniform fistula and to avoid unexpected occlusion of the fistula for the optimal experiment.
Initially, we tried to create an arterio-venous anastomosis between the pig iliac artery and iliac vein. However, we failed because the vessels were too deeply embedded and remotely separated. Consequently, the pig carotid artery and jugular vein were selected for a formation of AVF and they had a relatively superficial location for easier dissection and exposure, which provided the proper distance for an end to side anastomosis. Their diameters were enough for the free passages of the introducers, angiographic catheters, and balloon catheters. The technical success rate of the formation of AVFs was 92% and all the percutaneous procedures including angiograms and internal radiation therapy were successful. All of our pig models showed the strictures of the outflow veins on the angiograms taken 4 weeks after the formation of the AVFs and the strictures were located at the proximal outflow veins, which were very similar to those seen in human subjects.
Holmium-166, which was used as the radioactive substance in our study, is produced by the irradiation of neutrons to Holmium-165 in a nuclear reactor. Holmium-166 is made up of 95% beta rays (Emax = 1.84 MeV, half-life = 26.9 hours) and 5% gamma rays (0.081 Mev, 1.38 MeV), so it provides a powerful energy corresponding to Y-90. In addition, Holmium-166 has a mean radiation depth of 2.3 mm and then can be used safely without an injury to the adjacent tissue (12). Holmium-166 could be feasible radioactive material for the endovascular radiotherapy of AVF.
In the current study, the blood vessels of pigs were assumed to be cylindrical in shape and the radiation dose of Holmium-166 within 0-0.5 mm of the surface of target organ was calculated. The radiation dosimetry used in this study was measured by establishing the radiation source using a balloon catheter and then directly irradiating the target. To measure the radiation dose, the ionization chamber or thermoluminescence material were installed in the target area (1220). Based on these data, the amount and time of irradiation for the target radiation dose were determined (21). For the effective internal radiation therapy using radioactive isotope, it is essential to accurately measure the radiation dose. In the current study, Holmium-166 was irradiated within the blood vessels and the radiation dose was therefore accounted for based on the hypothesis that it is homogeneously distributed in the venous wall. However, it is impossible for the calculation of radiation dose to be accurately matched to human organs with no definite morphology (2223). To date, radioisotope-based reports about the treatment of tumor and arthritis have alluded to problems with the inaccuracies of radiation dosimetry. In most studies, the radiation dosimetry was identical to what was used for the previous experiences or other studies. For the clinical use of Holmium-166 in endovascular radiation therapy, fundamental studies about the measurement of radiation dose must be supported in various non-standardized human organ models.
In conclusion, the formation of an AVF between the carotid artery and jugular vein was successful in a pig model. The endovascular radiation using a Holmium-166 filled balloon catheter was technically feasible. Based upon the initial results of the current study, further studies to assure the safety and to depict the effectiveness upon the AVFs are mandatory for the human application of the endovascular radiation therapy using Holmium-166 filled balloon catheter.
Figures and Tables
Fig. 1
Photograph of the arteriovenous fistula in a pig. After cutting the distal end, internal jugular vein (arrow) was sutured to the common carotid artery using the end-to-side anastomosis method.
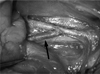
Fig. 2
Angiogram of the arteriovenous fistula of the pig. After placing a 5F catheter at the common carotid artery, 15 mL of contrast material was injected at a rate of 5 mL/second. Bead-like multifocal stenoses are noted in venous side just below the arteriovenous anastomosis site of the fistula.
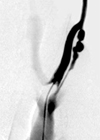
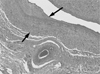
Fig. 3
Histologic section of venous limb of AVF with radiation (H & E stain, × 40). The section was performed at the venous segment with a stricture. Prominent neointimal hyperplasia with smooth muscle cell proliferation is seen (arrow). The maximal thickeness of the intima is about 1.2 mm.
Note.-AVF = arterio-venous fistula
References
1. Diskin CJ. Pharmacologic intervention to prevent hemodialysis vascular access thrombosis: the next generation of treatment? Kidney Int. 2005; 67:2505.
2. Windus DW. Permanent vascular access: a nephrologist's view. Am J Kidney Dis. 1993; 21:457–471.
3. Masaki T, Rathi R, Zentner G, Leypoldt JK, Mohammad SF, Burns GL, et al. Inhibition of neointimal hyperplasia in vascular grafts by sustained perivascular delivery of paclitaxel. Kidney Int. 2004; 66:2061–2069.
4. Quinn SF, Schuman ES, Demlow TA, Standage BA, Ragsdale JW, Green GS, et al. Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: intermediate results. J Vasc Interv Radiol. 1995; 6:851–855.
5. Sarac TP, Riggs PN, Williams JP, Feins RH, Baggs R, Rubin P, et al. The effects of low-dose radiation on neointimal hyperplasia. J Vasc Surg. 1995; 22:17–24.
6. Teirstein PS, Massullo V, Jani S, Russo RJ, Cloutier DA, Schatz RA, et al. Two-year follow-up after catheter-based radiotherapy to inhibit coronary restenosis. Circulation. 1999; 99:243–247.
7. Liermann DD, Bauernsachs R, Schopohl B, Böttcher HD. Five year follow-up after brachytherapy for restenosis in peripheral arteries. Semin Interv Cardiol. 1997; 2:133–137.
8. Johnson MS, McLennan G, Lalka SG, Whitfield RM, Dreesen RG. The porcine hemodialysis access model. J Vasc Interv Radiol. 2001; 12:969–977.
9. Trerotola SO, Carmody TJ, Timmerman RD, Bergan KA, Dreesen RG, Frost SV, et al. Brachytherapy for the prevention of stenosis in a canine hemodialysis graft model: preliminary observations. Radiology. 1999; 212:748–754.
10. Popma JJ, Suntharalingam M, Lansky AJ, Heuser RR, Speiser B, Teirstein PS, et al. Randomized trial of 90Sr/90Y beta-radiation versus placebo control for treatment of in-stent restenosis. Circulation. 2002; 106:1090–1096.
11. Grise MA, Massullo V, Jani S, Popma JJ, Russo RJ, Schatz RA, et al. Five-year clinical follow-up after intracoronary radiation: results of a randomized clinical trial. Circulation. 2002; 105:2737–2740.
12. Joh CW, Park CH, Kang HJ, Oh YT, Chun , Kim HS, et al. Measurement of radiation absorbed dose in endovascular Ho-166 brachytherapy using a balloon angio-catheter. Nucl Med Commun. 2000; 21:959–964.
13. Kanterman RY, Vesely TM, Pilgram TK, Guy BW, Windus DW, Picus D. Dialysis access grafts: anatomic location of venous stenosis and results of angioplasty. Radiology. 1995; 195:135–139.
14. Roy-Chaudhury P, Kelly BS, Narayana A, Desai P, Melhem M, Munda R, et al. Hemodialysis vascular access dysfunction from basic biology to clinical intervention. Adv Ren Replace Ther. 2002; 9:74–84.
15. Kim SJ, Masaki T, Leypoldt JK, Kamerath CD, Mohammad SF, Cheung AK. Arterial and venous smooth-muscle cells differ in their responses to antiproliferative drugs. J Lab Clin Med. 2004; 144:156–162.
16. Rodriguez VM, Grove J, Yelich S, Pearson D, Stein M, Pevec WC. Effects of brachytherapy on intimal hyperplasia in arteriovenous fistulas in a porcine model. J Vasc Interv Radiol. 2002; 13:1239–1246.
17. Malhotra S, Teirstein PS. The SCRIPPS trial--catheter-based radiotherapy to inhibit coronary restenosis. J Invasive Cardiol. 2000; 12:330–332.
18. King SB 3rd, Williams DO, Chougule P, Klein JL, Waksman R, Hilstead R, et al. Endovascular beta-radiation to reduce restenosis after coronary balloon angioplasty: results of the beta energy restenosis trial (BERT). Circulation. 1998; 97:2025–2030.
19. Waksman R, Robinson KA, Crocker IR, Gravanis MB, Cipolla GD, King SB 3rd. Endovascular low-dose irradiation inhibits neointima formation after coronary artery balloon injury in swine. A possible role for radiation therapy in restenosis prevention. Circulation. 1995; 91:1533–1539.
20. Kim HS, Cho YH, Kim JS, Oh YT, Kang HJ, Chun MS, et al. Effect of transcatheter endovascular radiation with holmium-166 on neointimal formation after balloon injury in porcine coronary artery. J Nucl Cardiol. 2000; 7:478–483.
21. Badal A, Kyprianou I, Badano A, Sempau J. Monte Carlo simulation of a realistic anatomical phantom described by triangle meshes: application to prostate brachytherapy imaging. Radiother Oncol. 2008; 86:99–103.
22. Kwok CS, Prestwich WV, Wilson BC. Calculation of radiation doses for nonuniformity distributed beta and gamma radionuclides in soft tissue. Med Phys. 1985; 12:405–412.
23. Kim JH, Lee JT, Kim EK, Won JY, Kim MJ, Lee JD, et al. Percutaneous sclerotherapy of renal cysts with a beta-emitting radionuclide, holmium-166-chitosan complex. Korean J Radiol. 2004; 5:128–133.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download