Figures and Tables
Fig. 1
Immunohistochemical staining of blood vessels. A. Each endothelial cell was stained with anti-CD31 antibody (arrows) in paraffin section. B. Thick section with CD31 immunostaining revealed relatively tube structure of blood vessels in cryosection (arrows). Magnification = × 200.

Fig. 2
The basis of optical sectioning and fluorescence. A. The confocal pinhole allows emission light from focal plane to reach the detector. Light from out-of-focus is eliminated by pinhole. B. The fluorophore absorbs energy of a specific wavelength and re-emit energy at a different wavelength. The excitation energy for a fluorophore can be delivered by multi-photon with relatively less energy in order to decrease fluorescent quenching.
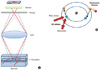
Fig. 3
Comparison of images in conventional microscopy (A) and confocal microscopy (B). Blood vessels in trachea are immunostained with anti-CD31 antibody. No significant difference were observed in both microscopy. Magnification = × 100.
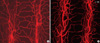
Fig. 4
Comparison of high-magnified images in conventional microscopy A. and confocal microscopy (B). (A) Only small area of blood vessels can be observed as in-focus image (CD31 immunostaining, red, × 200). B. All blood vessels in specimen can be obtained by three-dimensional reconstruction of each in-focus image (CD31 immunostaining, red; Ki-67 immunostaining, green, × 200). C. Cross-sectional image of tracheal blood vessels (CD31 immunostaining, red; Ki-67 immunostaining, green, × 50). D. High magnified view of endothelial proliferation in cross section (CD31 immunostaining, red; Ki-67 immunostaining, green, × 50).
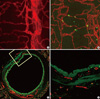
Fig. 5
Limitation of immunohistochemistry in thick specimens. A. Adipose tissue of mice with bone marrow transplanted from GFP mice. Endogenous GFP signals (green) are merged with macrophage staining (F4/80 immunostaining, blue, × 200). B. Optical sectioning images in series. Note that GFP signals could be detected in deeper area than immunostained signals (Perilipin immunostaining, red; F4/80 immunostaining, blue, × 200).

References
1. Ceska R. Clinical implications of the metabolic syndrome. Diab Vasc Dis Res. 2007. 4:S2–S4.
2. Arribas SM, Daly CJ, González MC, McGrath JC. Imaging the vascular wall using confocal microscopy. J Physiol. 2007. 584:5–9.
3. Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998. 70:2446–2453.
4. Suzuki H, Swei A, Zweifach BW, Schmid-Schönbein GW. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension. 1995. 25:1083–1089.
5. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003. 9:653–660.
6. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003. 9:669–676.
7. Minsky M. Memoir on inventing the confocal scanning microscope. Scanning. 1988. 10:128–138.
8. Dhaliwal JS, Kaufman SC, Chiou AG. Current applications of clinical confocal microscopy. Curr Opin Ophthalmol. 2007. 18:300–307.
9. Taylor AC, Seltz LM, Yates PA, Peirce SM. Chronic whole-body hypoxia induces intussusceptive angiogenesis and microvascular remodeling in the mouse retina. Microvasc Res. 2010. 79:93–101.
10. Erie JC, McLaren JW, Patel SV. Confocal microscopy in ophthalmology. Am J Ophthalmol. 2009. 148:639–646.
11. Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea - a major review. Clin Experiment Ophthalmol. 2009. 37:100–117.
12. Thiberville L, Salaün M, Lachkar S, Dominique S, Moreno-Swirc S, Vever-Bizet C, Bourg-Heckly G. Confocal fluorescence endomicroscopy of the human airways. Proc Am Thorac Soc. 2009. 6:444–449.
13. Goetz M, Memadathil B, Biesterfeld S, Schneider C, Gregor S, Galle PR, Neurath MF, Kiesslich R. In vivo subsurface morphological and functional cellular and subcellular imaging of the gastrointestinal tract with confocal mini-microscopy. World J Gastroenterol. 2007. 13:2160–2165.
14. Arribas SM, González JM, Briones AM, Somoza B, Daly CJ, Vila E, González MC, McGrath JC. Confocal myography for the study of hypertensive vascular remodelling. Clin Hemorheol Microcirc. 2007. 37:205–210.
15. Monici M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol Annu Rev. 2005. 11:227–256.
16. Pawley J. The Handbook Of Biological Confocal Microscopy. 1989. Madison, Wi, Usa: Imr Press.
17. van Zandvoort M, Engels W, Douma K, Beckers L, Oude Egbrink M, Daemen M, Slaaf DW. Two-photon microscopy for imaging of the (atherosclerotic) vascular wall: a proof of concept study. J Vasc Res. 2004. 41:54–63.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download