Abstract
Purpose
Various bone graft materials have been used for periodontal tissue regeneration. Demineralized freeze-dried bone allograft (DFDBA) is a widely used bone substitute. The current widespread use of DFDBA is based on its potential osteoinductive ability. Due to the lack of verifiable data, the purpose of this study was to assess the osteoinductive activity of different DFDBAs in vitro.
Methods
Sarcoma osteogenic (SaOS-2) cells (human osteoblast-like cells) were exposed to 8 mg/mL and 16 mg/mL concentrations of three commercial types of DFDBA: Osseo+, AlloOss, and Cenobone. The effect of these materials on cell proliferation was determined using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay. The osteoinductive ability was evaluated using alizarin red staining, and the results were confirmed by evaluating osteogenic gene expression using reverse transcription polymerase chain reaction (RT-PCR).
Results
In the SaOS-2 cells, an 8 mg/mL concentration of Osseo+ and Cenobone significantly increased cell proliferation in 48 hours after exposure (P<0.001); however, in these two bone materials, the proliferation of cells was significantly decreased after 48 hours of exposure with a 16 mg/mL concentration (P<0.001). The alizarin red staining results demonstrated that the 16 mg/mL concentration of all three tested DFDBA induced complete morphologic differentiation and mineralized nodule production of the SaOS-2 cells. The RT-PCR results revealed osteopontin gene expression at a 16 mg/mL concentration of all three test groups, but not at an 8 mg/mL concentration.
The ultimate goal of periodontal therapy is complete and predictable regeneration of lost periodontal tissue. Various bone graft materials have been used for periodontal tissue regeneration. Demineralized freeze-dried bone allograft (DFDBA) is a widely used bone substitute in periodontal and implant therapy. The pioneering studies of Urist [1] have shown that demineralization of bone results in enhanced osteogenic potential. DFDBA has been successfully used to reconstruct intraosseous periodontal defects and furcation defects. It has also been used in defects adjacent to dental implants to promote bone growth [2]. The current widespread use of DFDBA is based on the osteoinductive ability of this bone substitute. The demineralization process of the graft exposes the bone inductive proteins located in the bone matrix such as bone morphogenetic protein-2 (BMP2) and BMP7, which are capable of inducing mesenchymal cells to differentiate into osteoblasts in vivo [2,3]. DFDBA also provides an osteoconductive surface for cell attachment [4]. Despite the clinical success of DFDBA, some debate exists regarding its use in periodontal treatment, due in large part to the various reported results. Some studies have shown that variation in the osteoinductive activity of DFDBA depends on the donor characteristics (such as age, gender, and medical status), processing techniques, and particle size [2,5-8]. A direct clinical comparison of treatment success using FDBA and DFDBA yielded similar results [9]. Schwartz et al. [10] showed that some commercial preparations of DFDBA are inactive, due to a lack of adequate quantities of BMP. Other studies have questioned the continued use of unsupplemented DFDBA as an implant material for induction of bone adjacent to periodontal defects or dental implants [11]. Recently, several attempts have been made to establish a reliable method to assay osteoinductivity in vitro. A truly osteoinductive material must be capable of phenotypic conversion of progenitor cells within the healing wound to those that can form osseous tissue [12]. Due to the contradictory data and a lack of sensitive and reliable methods for screening the osteoinductivity of DFDBA, the purpose of this study was to assess the proliferation and differentiation of the Sarcoma osteogenic (SaOS-2) cell line (osteoblast-like cells) following exposure to different types of DFDBA.
The human osteoblast-like cell line (osteoblast-derived osteosarcoma cells) SaOS-2 (Pasteur Institute Cell Bank, Iran) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% antibiotic penicillin-streptomycin (Gibco) in an incubator with 5% CO2 at 37℃. The cells were seeded in culture plates of a DMEM medium with 10% FBS. After 24 hours of attachment, the medium was changed into a medium with 1% FBS. After 24 hours of incubation, the cells were treated with two concentrations (8 mg/mL and 16 mg/mL) of three different commercially available DFDBA powders (Table 1): Osseo+ (Imtec Co., Ardmore, OK, USA) (test group A), AlloOss (ACE Surgical Supply Co., Brockton, MA, USA) (test group B), and Cenobone (Tissue Regeneration Co., Kish, Iran) (test group C). The medium with 1% FBS was used as the negative control. The medium with 10% FBS and the osteogenic medium (containing dexamethasone 100 nmol/L, vitamin C 50 µg/mL, and -β glycerophosphate 10 mmol/L) with 1% FBS were used as the positive controls for proliferation and differentiation, respectively.
In first stage, for measuring the optimum dosage of DFDBA, the SaOS-2 cells were plated in 6-well plates at a density of 1×104 cells/well. The cells were treated with five concentrations (4, 8, 12, 16, and 20 mg/mL) of three different commercially available DFDBA powders. The cells were incubated for 24 hours after treatment, and morphologic (apoptotic changes) assessment was performed using microscopy (Nikon, Tokyo, Japan) under ×10-×40 magnification. The results showed that a 20 mg/mL concentration had a toxic effect on the SaOS-2 cells, and under the 4, 8, 12, and 16 mg/m concentrations, cells had normal morphologic features. Thus, 8 and 16 mg/mL concentrations were chosen for this study.
The cell proliferation and viability were evaluated 24 and 48 hours after exposure to three test group powders using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich Co., St. Louis, MO, USA). The cells were incubated 24 and 48 hours after treatment, and then exposed with a MTT solution of 3-(4,5-dimethylthiazol-2-yl)-2 and 5-diphenyltetrazolium bromide at 0.5 mg/mL for 1 hour at 37℃. After the incubation period, the formazan crystals were dissolved in dimethyl sulfoxide and transferred to a 96-well plate in triplicate. The optical density was measured on a microplate reader (Anthos Labtec Instruments GmbH, Wals-Siezenheim, Austria) at 570 nm, with 690 nm as a reference wavelength. The change in viability was calculated as percent viability compared to the control group, and the results of the three independent experiments were presented as mean±standard deviation (SD) (n=3).
The formation of calcium phosphate by SaOS-2 osteoblast-like cells was determined by using an alizarin red-S assay (Sigma-Aldrich Co.). For evaluating cell differentiation, the SaOS-2 cells were plated in 60 mm petri dishes at a density of 2×104 cells. On day 5 after the treatment, the medium was removed and the cells were washed with phosphate buffer saline 3 times and fixed with ice-cold ethanol at room temperature for 60 minutes. The fixed cells were stained with 2% alizarin red-S (pH 4.2) for 15 minutes at room temperature. The cells were then washed with deionized water 4 times, and orange/red nodules were observed by microscopy (Nikon) under ×10-×40 magnification.
Expression of specific osteogenic differentiation genes including osteonectin (ON), osteopontin (OP), and osteocalcin (OC) was assessed qualitatively on days 3 and 5 of the experiment in both the 8 mg/mL and 16 mg/mL DFDBA groups using RT-PCR. For osteogenic gene analysis, the SaOS-2 cells were plated in 60 mm plates at a density of 2×104 cells. The total RNA was extracted from the treated cells using the RNeasy Kit (RNeasy, Qiagen, Valencia, CA, USA) and cDNA was synthesized from 1 µg of the total RNA using a cDNA synthesis kit (Bioneer Co., Daejeon, Korea) according to the manufacturer's instructions. The OC, ON, and OP mRNA expression was evaluated using RT-PCR with specific primers (Table 2). The PCR was performed using the following process: 95℃ for 2 minutes, 35 cycles of denaturation at 95℃ for 30 seconds, annealing for 30 seconds (Table 2), extension at 72℃ for 30 seconds, and a final extension at 72℃ for 5 minutes. The β-actin was used as a housekeeping gene (internal control). The PCR products were analyzed on 1.8% agarose gels and ultraviolet illumination.
Each experiment was repeated 2 times using two different batches of each type of DFDBA, which were selected randomly.
MTT assay data are presented as the means±SD of at least three independent experiments. Statistical analysis was performed using one way analysis of variance followed by Tukey-Kramer multiple comparison, and P-values less than 0.05 were considered statistically significant. The results obtained from RT-PCR analysis and alizarin red staining were assessed qualitatively.
In 24 hours after treatment, cell proliferation and viability of the three experimental groups in both concentrations (8 and 16 mg/mL) were significantly lower than the negative control (P<0.001). Cell proliferation and viability at 8 mg/mL concentration and at 16 mg/mL were 68% and 91% in test group A (Osseo+), 88% and 84% for test group B (AlloOss), and 61% and 81% for test group C (Cenobone), respectively (Fig. 1A).
In 48 hours after the three DFDBA treatments, cell proliferation and viability were significantly (P<0.001) lower than the negative control in test groups A and C, which had a 6% and 10% decrease at 8 mg/mL concentration, respectively, and a 20% and 31% decrease at 16 mg/mL concentration, respectively. However, the difference between test group B (in both concentrations) and the negative control was not significant (P>0.05). At this point in time, only the differences between the AlloOss and Cenobone group at 8 mg/mL concentration were significant (P<0.001) (134% vs. 90%, respectively). However, at the 16 mg/mL concentration, the differenes among the three experimental groups was significant (P<0.001) (80% for the Osseo+ group, 99% for the AlloOss group, and 69% for the Cenobone group) (Fig. 1B).
The results of alizarin red staining 5 days after DFDBA treatment showed that morphologic differentiation to osteoblasts and formation of mineralized nodules occurred in all three experimental groups at the 16 mg/mL concentration, but did not occur at the 8 mg/mL concentration. The differentiated SaOS-2 cells in the positive control group and in all three test groups at 16 mg/mL concentration lost their primary polyhedral shape with the round central nucleus, which was typically seen in the undifferentiated SaOS-2 cells of the negative control, and they acquired an elongated morphology with long osteoblastic processes and a polarized spindle-shaped nucleus. As shown in Fig. 2, the red and orange calcification nodules were observed in the positive control group and in all three test groups at the 16 mg/mL concentration, but were not observed in the negative control group or in all three test groups at the 8 mg/mL concentration.
To investigate the effects of DFDBA powders on early and late mRNA expression of osteogenic markers (OP, ON, and OC), we evaluated the expression of these genes on days 3 and 5 after treatment with RT-PCR analysis. Expression of β-actin gene as an internal control for RT-PCR was positive and similar in all the test groups. As shown in Fig. 3A and B, the ON and OC gene expressed in all three experimental groups and in the two control groups at both concentrations. However, OP gene expression was only seen in the three experimental groups at 16 mg/mL concentration and in the positive control, but no expression was observed in the negative control group or in any of the three test groups at 8 mg/mL concentration in the SaOS-2 cells (Fig. 3A), suggesting a dose-dependent osteoinductive effect of these materials on these cells.
Due to the existing contradictory results on the osteoinductive activity of DFDBA, the purpose of this study was to assess the proliferation and differentiation (osteoinductive activity) of SaOS-2 cells (the human osteoblast-like cell line) following exposure to three different commercially available types of DFDBA. The results of alizarin red staining showed that morphologic differentiation to osteoblasts and formation of mineralized nodules occurred in all three of the experimental groups at the 16 mg/mL concentration. However, a few of the differentiated cells and calcium nodules were observed at the 8 mg/mL concentration. The present study was the first study that used this specific staining method for assessing the osteoinductive properties in this cell line.
Lian and Stein [13] and Rodan et al. [14] reported that when proliferation decreased in the SaOS-2 cells, the expression of the osteoblastic functions, including alkaline phosphatase activity and extracellular matrix (ECM) production, increased. Comparing the cell proliferation rate between 24 and 48 hours after DFDBA treatment showed that the proliferation rate had significantly increased at the 8 mg/mL concentration in all of the experimental groups, while it had significantly decreased in the Osseo+ and Cenobone groups at the 16 mg/mL concentration. This means that in vitro osteoinductive activity of DFDBA may be dose-dependent, such that in the 16 mg/mL concentration, the proliferation rate decreased in shorter periods of time, and the cells were differentiated to osteoblasts after 3 days of exposure.
Carnes et al. [15] demonstrated that 2T9 cells exhibited a dose-dependent response to soluble BMP2, and in the presence of BMP2, proliferation of these cells decreased, and alkaline phosphatase activity, OC production, and mineralized nodule formation increased. Decreased proliferative activity of bone marrow stem cells following exposure to DFDBA has been reported in a study by Kumaran et al. [12]. Bormann et al. [3] also showed that the proliferation rate of the C2C12 cell line decreased following exposure to two commercial types of DFDBA including Allomatrix and demineralized bone matrix putty. The proliferation rates in our study are in agreement with the above studies.
Rochet et al. [16] and Trojani et al. [17] demonstrated that the ON and OC gene primarily express in the SaOS-2 cell line, but the OP gene does not express in these cells. Thus expression of OP is a critical marker for terminal osteoblastic differentiation of the SaOS-2 cell line. Our study also confirmed those findings. Our results showed that OC and ON genes expressed in all the groups. However, OP gene expression was observed only at the 16 mg/mL concentration in all of the three experimental groups, but not at the 8 mg/mL concentration, suggesting an in vitro dose-dependent osteoinductive effect of these materials on this cell line. The RT-PCR results demonstrated osteoinductive activity of these 3 commercial types of DFDBA and confirmed the alizarin red staining results.
In most of the previous studies for evaluation of osteoinductive activity in vitro, only alkaline phosphatase activity assay had been used [3,12,18]. The use of RT-PCR and evaluation of osteogenic markers increases the strength of a study that is assessing the osteoinduction properties [15,19]. Kumaran et al. [12] and Bormann et al. [3] reported on the alkaline phosphatase activity of bone marrow stem cells and the C2C12 cell line following exposure to DFDBA. Han et al. [18] demonstrated that alkaline phosphatase activity of the C2C12 cell line varied widely from bank to bank as well as from batch to batch within the same bank.
The results of the present study are in agreement with Carinci et al. [19], in which the authors identified, in an MG-63 cell line (osteoblast-like cell) cultured with DFDBA, that expression of several genes, such as those responsible for ECM component formation and bone metabolism and remodeling, were significantly up-regulated. In contrast to our results, Carnes et al. [15] reported that when 2T9 cells were exposed to DFDBA in the presence or absence of BMP2, no effect on 2T9 cell differentiation was observed. Their study showed that DFDBA released no soluble factors with bone inductive ability and that if any active factors were adsorbed to the DFDBA, they were inactive. One reason for this may be the type of cells used and another may be the cytotoxicity of selected concentrations of DFDBA for 2T9 cells.
In future studies, the use of bone marrow stem cells could be beneficial for observing the effects of the osteoinductive properties on noncommitted osteoprogenitor cells such as myeloid and lymphoid stem cells. Adding qualitative assessments using real-time PCR and assessing protein production with western blot assay would also contribute toward improving the validity of the present study.
In conclusion, our study indicates that these three commercially available types of DFDBA are capable of decreasing proliferation and increasing osteogenic differentiation of the SaOS-2 cell line in 16 mg/mL concentrations and have osteoinductive activity in vitro.
Figures and Tables
Figure 1
The effect of A, B and C test groups on saos-2 cell viability and proliferation. Cells were treated with 8 mg/mL (A) and 16 mg/mL (B) concentration of test groups. After 24 and 48 hours, the cell viability and proliferation were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The results are presented as mean±standard deviation (SD) of three independent experiments (n=3; a)p<0.001, b)P<0.01).

Figure 2
The effect of test groups A, B and C on bone mineralization in sarcoma osteogenic (SaOS-2) cell. Optical image of alizarin red S in SaOS-2 osteoblast-like cells for day 5 after treatment with 8 mg/mL and 16 mg/mL concentration of test groups.
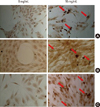
Figure 3
The effect of A, B and C test groups on expression of osteogenic markers in sarcoma osteogenic (SaOS-2) cells. SaOS-2 cells were treated with 8 mg/mL (A) and 16 mg/mL (B) concentration of test groups. The mRNA expression of 3 osteogenic markers was evaluated 3 and 5 day after treatment and compared with positive (differentiated cells) and negative control (undifferentiated cells). β-actin was used as internal control. OP: osteopontin, OC: osteocalcin, ON: osteonectin.

ACKNOWLEDGEMENTS
This research was supported by a research grant from the Tissue Regeneration Corporation. The authors also wish to express their gratitude to Ms. Bahareh Sharifi for technical assistance and to Dr. Fahimeh Sadat Tabatabaei for helpful advice (Molecular and Cellular Oral Biology Laboratory, Shahid Beheshti University of Medical Sciences, Tehran, Iran).
References
1. Urist MR. Bone histogenesis and morphogenesis in implants of demineralized enamel and dentin. J Oral Surg. 1971. 29:88–102.
2. Schwartz Z, Mellonig JT, Carnes DL Jr, de la Fontaine J, Cochran DL, Dean DD, et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol. 1996. 67:918–926.

3. Bormann N, Pruss A, Schmidmaier G, Wildemann B. In vitro testing of the osteoinductive potential of different bony allograft preparations. Arch Orthop Trauma Surg. 2010. 130:143–149.

4. Zhang M, Powers RM Jr, Wolfinbarger L Jr. A quantitative assessment of osteoinductivity of human demineralized bone matrix. J Periodontol. 1997. 68:1076–1084.

5. Shigeyama Y, D'Errico JA, Stone R, Somerman MJ. Commercially-prepared allograft material has biological activity in vitro. J Periodontol. 1995. 66:478–487.

6. Pinholt EM, Haanaes HR, Roervik M, Donath K, Bang G. Alveolar ridge augmentation by osteoinductive materials in goats. Scand J Dent Res. 1992. 100:361–365.

7. Becker W, Lynch SE, Lekholm U, Becker BE, Caffesse R, Donath K, et al. A comparison of ePTFE membranes alone or in combination with platelet-derived growth factors and insulin-like growth factor-I or demineralized freeze-dried bone in promoting bone formation around immediate extraction socket implants. J Periodontol. 1992. 63:929–940.

8. Becker W, Schenk R, Higuchi K, Lekholm U, Becker BE. Variations in bone regeneration adjacent to implants augmented with barrier membranes alone or with demineralized freeze-dried bone or autologous grafts: a study in dogs. Int J Oral Maxillofac Implants. 1995. 10:143–154.
9. Rummelhart JM, Mellonig JT, Gray JL, Towle HJ. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1989. 60:655–663.

10. Schwartz Z, Somers A, Mellonig JT, Carnes DL Jr, Wozney JM, Dean DD, et al. Addition of human recombinant bone morphogenetic protein-2 to inactive commercial human demineralized freeze-dried bone allograft makes an effective composite bone inductive implant material. J Periodontol. 1998. 69:1337–1345.

11. Becker W, Urist MR, Tucker LM, Becker BE, Ochsenbein C. Human demineralized freeze-dried bone: inadequate induced bone formation in athymic mice. A preliminary report. J Periodontol. 1995. 66:822–828.

12. Kumaran ST, Arun KV, Sudarsan S, Talwar A, Srinivasan N. Osteoblast response to commercially available demineralized bone matrices: an in-vitro study. Indian J Dent Res. 2010. 21:3–9.

13. Lian JB, Stein GS. The developmental stages of osteoblast growth and differentiation exhibit selective responses of genes to growth factors (TGF beta 1) and hormones (vitamin D and glucocorticoids). J Oral Implantol. 1993. 19:95–105.
14. Rodan SB, Imai Y, Thiede MA, Wesolowski G, Thompson D, Bar-Shavit Z, et al. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987. 47:4961–4966.
15. Carnes DL Jr, De La Fontaine J, Cochran DL, Mellonig JT, Keogh B, Harris SE, et al. Evaluation of 2 novel approaches for assessing the ability of demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol. 1999. 70:353–363.

16. Rochet N, Loubat A, Laugier JP, Hofman P, Bouler JM, Daculsi G, et al. Modification of gene expression induced in human osteogenic and osteosarcoma cells by culture on a biphasic calcium phosphate bone substitute. Bone. 2003. 32:602–610.

17. Trojani C, Weiss P, Michiels JF, Vinatier C, Guicheux J, Daculsi G, et al. Three-dimensional culture and differentiation of human osteogenic cells in an injectable hydroxypropylmethylcellulose hydrogel. Biomaterials. 2005. 26:5509–5517.

18. Han B, Tang B, Nimni ME. Quantitative and sensitive in vitro assay for osteoinductive activity of demineralized bone matrix. J Orthop Res. 2003. 21:648–654.

19. Carinci F, Piattelli A, Degidi M, Palmieri A, Perrotti V, Scapoli L, et al. Effects of demineralized freeze-dried bone allograft on gene expression of osteoblastlike MG63 cells. Int J Periodontics Restorative Dent. 2007. 27:596–601.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download