Abstract
Purpose
The aim of this study was to evaluate the improvement of osteogenic potential of biphasic calcium phosphate (BCP) bone substitute coated with synthetic cell-binding peptide sequences in a standardized rabbit sinus model.
Methods
Standardized 6-mm diameter defects were created bilaterally on the maxillary sinus of ten male New Zealand white rabbits, receiving BCP bone substitute coated with synthetic cell binding peptide sequences on one side (experimental group) and BCP bone substitute without coating (control group) on the other side. Histologic and histomorphometric analysis of bone formation was carried out after a healing period of 4 or 8 weeks.
Results
Histological analysis revealed signs of new bone formation in both experimental groups (4- and 8-week healing groups) with a statistically significant increase in bone formation in the 4-week healing group compared to the control group. However, no statistically significant difference in bone formation was found between the 8-week healing group and the control group.
Various bone substitutes have been used for sinus floor elevation procedures. Autologous bone is considered the gold standard [1-3]. However, the autologous bone transplanted may have an inconsistent rate of mineralization, due to the ratio of cortical to cancellous bone, and given that it also requires an additional donor-site surgery, it may not thus always be the material of choice for sinus floor elevation [3].
Recently, biphasic calcium phosphate (BCP) bone substitute has been investigated and used in sinus floor elevation procedures with favorable treatment outcomes [4-6]. BCP is a proven safe and biocompatible synthetic bone substitute that is widely used in orthopedic, maxillofacial, and periodontal surgery [7,8]. BCP is known to have mainly osteoconductive properties [8]. Therefore, there have been various efforts to improve the osteogenic potential of this BCP with growth factor, recombinant human bone morphogenetic protein (rhBMP)-2 [9,10]. Previous studies using BCP treated with rhBMP-2 on rat calvarial defects have shown improved osteoinductive potentials when compared with untreated BCP. However, considering side effects such as the swelling of soft tissues and ectopic bone and cyst-like bone void formation, rhBMP-2 should not be applied to all periodontal defects or used in sinus floor elevation [11-13].
Biologically active peptides have been bound to the surface of inorganic bone substitutes to improve the performance of biomaterial with limited osteoinductive potentials [14-16]. PepGen (P-15) is a Food and Drug Administration approved tissue engineered product that contains a synthetic 15 amino acid sequence (766GTPGPQGIAGQRGVV780) of the α1-chain in Type I collagen, which is uniquely involved in binding of cells such as fibroblasts or osteoblasts [17-19]. In previous studies, it was shown that there was an increase in the attachment and viability of cells bound to an inorganic bone substitute with P-15 when compared with those without P-15 [20,21]. Favorable results in animal and clinical studies were also reported when using inorganic bone substitute with P-15 [14, 22]. However, binding of this biologically active peptide to BCP has not yet been attempted or investigated thoroughly. Therefore, the purpose of this study was to evaluate the improvement of osteogenic potential of BCP coated with synthetic peptide (amino acid sequence of 766GTPGPQGIAGQRGVV780).
Standardized, bilateral, circular, transosseous, 6-mm diameter defects were created on the maxillary sinuses of the subjects. The bone graft material used in this study was BCP bone substitute, Osteon (Dentium Co., Seoul, Korea), which consists of 70% hydroxyapatite and 30% β-TCP (Table 1).
A peptide sequence of 766GTPGPQGIAGQRGVV780 was obtained from the manufacturer (Sigma-Aldrich Japan, Ishikari, Japan). Surface coating was then performed to combine hydroxyapatite, a constituent of BCP (Osteon, Dentium Co.), with the peptide. At first, the hydroxyl group of hydroxyapitite was linked with 3-aminopropyltriethoxysilane (Sigma-Aldrich Co., St. Louis, MO, USA), then linked with N-succinimidyl 3-maleimidopropionate (Sigma-Aldrich Co.), followed by a linkage with the peptide sequence. The BCP was soaked in the peptide solution and stored at -70℃ in a deep-freezer. To sublimate the liquid phase and avoid denaturalizing the peptide, the frozen solution was then placed for a day in a vial in a lyophilizer (Ilshin Lab., Seoul, Korea). The lyophilization setting was put at -45℃ to -50℃ with a high vacuum of 7 to 10 mTorr, and the dried granules were kept in a freezer until use. The peptide-coated BCP was sterilized with ethylene oxide (EO) gas in a gas sterilizer (Steri-Vac 400B, 3M, St. Paul, MN, USA) at 29℃ for 5 hours; this is considered the ideal sterilization protocol without hampering osteoinductive activity [23].
Ten male New Zealand white rabbits (body weight, 2.5 to 3.0 kg) were used for this study. The window position on the maxillary sinus of rabbits was determined according to the instructions of Asai et al. [24]: 20 mm anterior to the nasofrontal suture line and 10 mm lateral to the midline.
Prior to all surgical procedures, the animals were anesthetized with a cocktail of ketamine hydrochloride (Ketalar, Yuhan, Seoul, Korea) and xylazine (Rumpun, Bayer Korea Ltd., Seoul, Korea) via an intramuscular injection. The surgical site was then shaved and sterilized with a povidon-iodine solution, followed by local anesthesia of 2% lidocaine with 1:100,000 epinephrine. After making a straight incision along the sagittal midline on the nasal bone, a full-thickness flap was reflected, exposing the calvarial bone. A standardized, circular, bilateral, transosseous window was created on the maxillary sinus using 6-mm-diameter trephine bur while being irrigated copiously with saline. A pin was also inserted at the mid-point between the two windows to indicate the reference point for micro-computed tomography (CT) analysis. Following removal of the trephined calvarial disk, the experimental and control treatments of grafting material were applied to the windows accordingly (Table 1). The periosteum was then repositioned over the windows and all surgical sites were sutured with 4-0 Monosyn (Glyconate absorbable monofilament, B-Braun, Aesculap, Center Valley, PA, USA). The animals were euthanized 4 or 8 weeks after the surgery.
The harvested block sections, including the augmented sinus and the surrounding bone, were fixed in 10% buffered formalin for 10 days and scanned using micro-CT (SkyScan 1076, SkyScan, Aartselaar, Belgium) at a resolution of 35 µm (100 kV and 100 µA). The area of interest was reconstructed with Ondemand 3D software (Cybermed, Seoul, Korea) and rendered visually in 3D. The CT volume was calculated from the percentage of window closure using an automated image-analysis system (Image-Pro Plus, Media Cybernetics Inc., Silver Spring, MD, USA).
After the micro-CT image was obtained, each of the sections was decalcified in 5% formic acid for 14 days and then embedded in paraffin. Serial 5-µm-thick sections were cut coronally along the center of the circular calvarial defects. The 2 central-most sections were selected and stained with hematoxylin and eosin (H&E) for light microscope examination (BX50, Olympus Co., Tokyo, Japan).
An automated image-analysis system (Image-Pro Plus, Media Cybermetics Inc.) was used for computer-assisted histometric analysis. Two parameters were measured: newly formed bone area (NB%) and remaining graft area (RG%). NB% was measured by calculating newly formed mineralized bone in the window as a percentage of total augmented bone area, measured as all tissues within the boundary of the newly formed bone. RG% was measured by calculating the area of the remaining grafting materials as a percentage of the total augmented bone area.
Micro-CT data and histomorphometric measurement of the samples were used to calculate group mean±SD values. The Mann-Whitney U test was performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) to compare differences between the control group and the experimental group, as well as differences between two experimental groups according to time of healing. A P-value less than 0.05 was considered statistically significant.
In the 4-week healing group, NB% was statistically significantly greater in the experimental group than in the control group (P<0.05). RG% was less in the experimental group than in the control group. However, no statistically significant difference was found in the control and experimental groups' RG% values. In the 8-week healing group, while CT volume was statistically significantly greater in the experimental group than in the control group (P<0.05), no statistical difference was found in NB%, despite its increased value (Table 2).
Histological findings revealed that increased new bone formation was particularly evident in the experimental group (BCP coated with oligopeptide) when compared to that of the control group (BCP alone). Additionally, more osteoblasts and osteocytes in lacunae were identified in the experimental group (Figs. 1 and 2). In general, within the experimental group, more vascularization and the presence of newly formed blood vessels were identified in the 4-week healing group. Immature woven bone, still undergoing the mineralization process, was found to be dominant in the 4-week healing group relative to the 8-week healing group (Fig. 3).
In contrast, the Schneiderian membrane was slightly thickened in the 8-week healing group. The shape of the graft material was also smoothened, which indicates the resorption of graft material particles (Fig. 4). In between graft material particles, Haversian systems and interstitial lamella were observed, both of which are indicative of matured lamellar bone (Fig. 5).
There have been many attempts to increase the osteoinductivity of bone graft materials by using a variety of growth factors. The use of BMPs, in particular, has shown favorable results in promoting bone formation [25] and has therefore been investigated to assess its effectiveness through various animal models [26]. However, the use of BMPs also appears to be difficult to handle due to their high molecular weight, the technical difficulties in binding them to bone graft particles, their relatively high cost, and the complexity of immunologic responses [27,28]. Moreover, there is also the limiting factor of establishing an optimum therapeutic concentration of BMPs; since various unwanted side-effects have been reported, such as ectopic bone formation, cyst-like bone void formation, and soft tissue swelling when supraphysiologic doses of BMPs were used [11,12].
Because of this unpredictability, receptor technologies, particularly the use of specific peptide sequences (oligopeptide) involved in binding to cells such as fibroblasts or osteoblasts, have been posited as alternatives. Saito et al. [29] have reported that 73 to 92 peptide combined with the porous a-TCP scaffold almost completely repaired a 20-mm long rabbit radial bone defect 12 weeks after implantation. Suzuki et al. [30] have also shown that alginate hydrogel linked with synthetic oligopeptiode derived from BMP-2 promoted ectopic bone formation. Among many peptide sequences, the use of P-15 in particular, which includes synthetic 15 peptide sequence (766GTPGPQGIAGQRGVV780) of the α1-chain in Type I collagen, has already shown favorable results in the treatment of periodontal defects and in sinus-lifting procedures [14,22]. Park et al. [14] have also reported that 15 amino acids corresponding to the BMP I and II receptor-binding domains coated on granules of bovine bone mineral significantly accelerated new bone formation in a rabbit calvarial defect model. The use of this oligopeptide corresponding to the BMP receptor-binding domains also proved effective in enhancing the bone regenerative capacity in a beagle dog's 1-wall intrabony defect model [31].
To date, there have been many studies attempting to increase the osteoinductive potential of BCP, one of the most widely used bone graft materials, by coating it with BMPs. However, the effects of binding a biologically active peptide to BCP to increase osteoinductive potential have not been investigated thoroughly. Therefore, the purpose of the present study was to evaluate the improvement of osteogenic potential of BCP coated with synthetic peptide (amino acid sequence of 766GTPGPQGIAGQRGVV780).
In order to provide precisely the same conditions to the control and the experimental group and to minimize procedural errors, the experiment conducted in the study used the standardized rabbit sinus model with the same window shape and size introduced by De Souza Nunes et al. [32]. According to Roberts and Breznak [33], the rabbit metabolic rate is known to be three to four times faster than that of the human. Therefore healing periods of 4 and 8 weeks were chosen based on the assumption that a 4-week and an 8-week healing period for rabbits would correspond to a 3- to 4-month and a 6- to 8-month healing period in humans, respectively.
From histomorphometric analysis, it was found that in the 4-week healing group, new bone formation was statistically significantly greater in the experimental group than the control group. This result is in accordance with previous studies [34-36], as it was thought that bone graft material coated with oligopeptide would promote cell adhesion and cell differentiation, providing more favorable conditions for new bone formation. CT volume was also statistically significantly increased in the experimental group than the control group, which also supports the idea that bone graft materials with oligopeptide coating have more osteoinductive potential. However, in the 8-week healing group, though both new bone formation and CT volume in the experimental group were greater than those of the control group, there was no statistically significant difference in bone formation or CT volume between the 4-week healing group and the 8-week healing group.
Park et al. [14] also reported that oligopeptide domain-coated bovine bone mineral promoted new bone formation at 2 and 4 weeks postsurgery. The results of the current study show the effect of oligopeptide on early bone formation is clearly evident in the 4-week healing group but not as notable in the 8-week healing group. Perhaps, then, it can be suggested that oligopeptide coating may be more beneficial for bone regeneration in the early healing period. On the other hand, the current results may also be due to a high standard deviation resulting from a limited sample size. In this case, further studies with a greater sample size may help to better determine the effectiveness of oligopeptide coating according to healing time.
Within the experimental group, the 8-week healing group showed greater bone formation than did the 4-week healing group, which demonstrates that the process of bone formation continued to occur between the 4th and 8th weeks. For this reason, it may be beneficial to observe the bone formation process even after an 8-week period of time in order to accurately determine the effect of the oligopeptide coating on healing time. It is interesting to note that CT volume in the 8-week healing group was lower. One interpretation of this is that the replacement of biomaterial with newly formed bone does not necessarily occur at a 1:1 ratio, which is in agreement with Frenken et al. [5]. In other words, less bone is produced in this case, compared to the volume of biomaterials resorbed.
In conclusion, this study found that BCP bone substitute coated with biologically active peptide sequences enhanced osteoinductive potential in a standardized rabbit sinus model and, within the limitations of this study, it can be assumed that oligopeptide coating is more beneficial for bone regeneration in the early healing period.
Figures and Tables
Figure 1
Histological findings of control group (biphasic calcium phosphate) at 4 weeks. (A) Area of interest (H&E, ×12.5), (B) defect margin area (H&E, ×50), (C) the Schneiderian membrane (H&E, ×50), and (D) middle area (H&E, ×50).
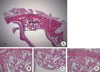
Figure 2
Histological findings of experimental group (biphasic calcium phosphate coated with oligopeptides) at 4 weeks. (A) Area of interest (H&E, ×12.5), (B) defect margin area (H&E, ×50), (C) the Schneiderian membrane (H&E, ×50), and (D) middle area (H&E, ×50).
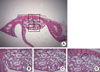
Figure 3
Histological findings of the Schneiderian membrane in experimental group (biphasic calcium phosphate coated with oligopeptides) at 4 weeks. The Schneiderian membrane (H&E, ×50). More vascularization (white asterisks) with newly formed blood vessels (arrows) were identified in the 4-week healing group. Bone matrix, including osteoblasts (arrowheads), was also observed.
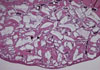
Figure 4
Histological findings of experimental group (biphasic calcium phosphate coated with oligopeptides) at 8 weeks. (A) Area of interest (H&E, ×12.5), (B) defect margin area (H&E, ×50), (C) the Schneiderian membrane (H&E, ×50), and (D) middle area (H&E, ×50). The Schneiderian membrane was thicker than that of the 4-week healing group. Also, the shape of graft materials was smoothened, which indicates the resorption of graft material particles.
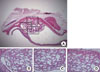
Figure 5
Histological findings of middle area in experimental group (biphasic calcium phosphate coated with oligopeptides) at 8 weeks. Middle area (H&E, ×50). Haversian systems (arrowheads) and interstitial lamella (black asterisks) were observed, both of which are indicative of matured lamellar bone.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2011-0004172).
References
1. Klijn RJ, Meijer GJ, Bronkhorst EM, Jansen JA. A meta-analysis of histomorphometric results and graft healing time of various biomaterials compared to autologous bone used as sinus floor augmentation material in humans. Tissue Eng Part B Rev. 2010. 16:493–507.

2. Klijn RJ, Meijer GJ, Bronkhorst EM, Jansen JA. Sinus floor augmentation surgery using autologous bone grafts from various donor sites: a meta-analysis of the total bone volume. Tissue Eng Part B Rev. 2010. 16:295–303.

3. Schlegel KA, Schultze-Mosgau S, Wiltfang J, Neukam FW, Rupprecht S, Thorwarth M. Changes of mineralization of free autogenous bone grafts used for sinus floor elevation. Clin Oral Implants Res. 2006. 17:673–678.

4. Schmitt CM, Doering H, Schmidt T, Lutz R, Neukam FW, Schlegel KA. Histological results after maxillary sinus augmentation with Straumann® BoneCeramic, Bio-Oss®, Puros®, and autologous bone. A randomized controlled clinical trial. Clin Oral Implants Res. 2012. 02. 13. [Epub]. http://dx.doi.org/10.1111/j.1600-0501.2012.02431.x.

5. Frenken JW, Bouwman WF, Bravenboer N, Zijderveld SA, Schulten EA, ten Bruggenkate CM. The use of Straumann Bone Ceramic in a maxillary sinus floor elevation procedure: a clinical, radiological, histological and histomorphometric evaluation with a 6-month healing period. Clin Oral Implants Res. 2010. 21:201–208.

6. Cordaro L, Bosshardt DD, Palattella P, Rao W, Serino G, Chiapasco M. Maxillary sinus grafting with Bio-Oss or Straumann Bone Ceramic: histomorphometric results from a randomized controlled multicenter clinical trial. Clin Oral Implants Res. 2008. 19:796–803.

7. Kim S, Jung UW, Lee YK, Choi SH. Effects of biphasic calcium phosphate bone substitute on circumferential bone defects around dental implants in dogs. Int J Oral Maxillofac Implants. 2011. 26:265–273.
8. Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med. 2003. 14:195–200.
9. Kim JW, Choi KH, Yun JH, Jung UW, Kim CS, Choi SH, et al. Bone formation of block and particulated biphasic calcium phosphate lyophilized with Escherichia coli-derived recombinant human bone morphogenetic protein 2 in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011. 112:298–306.

10. Park JC, So SS, Jung IH, Yun JH, Choi SH, Cho KS, et al. Induction of bone formation by Escherichia coli-expressed recombinant human bone morphogenetic protein-2 using block-type macroporous biphasic calcium phosphate in orthotopic and ectopic rat models. J Periodontal Res. 2011. 46:682–690.

11. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008. 8:1011–1018.

12. Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, et al. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2000. 27:479–486.

13. Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Phila Pa 1976). 2006. 31:2813–2819.

14. Park JB, Lee JY, Park HN, Seol YJ, Park YJ, Rhee SH, et al. Osteopromotion with synthetic oligopeptide-coated bovine bone mineral in vivo. J Periodontol. 2007. 78:157–163.

15. Nam HW, Park JB, Lee JY, Rhee SH, Lee SC, Koo KT, et al. Enhanced ridge preservation by bone mineral bound with collagen-binding synthetic oligopeptide: a clinical and histologic study in humans. J Periodontol. 2011. 82:471–480.

16. Kim TI, Jang JH, Lee YM, Rhyu IC, Chung CP, Han SB, et al. Biomimetic approach on human periodontal ligament cells using synthetic oligopeptides. J Periodontol. 2004. 75:925–932.

17. Hagel-Bradway S, Dziak R. Regulation of bone cell metabolism. J Oral Pathol Med. 1989. 18:344–351.

18. Seyedin SM. Osteoinduction: a report on the discovery and research of unique protein growth factors mediating bone development. Oral Surg Oral Med Oral Pathol. 1989. 68(4 Pt 2):527–529.

19. Hole BB, Schwarz JA, Gilbert JL, Atkinson BL. A study of biologically active peptide sequences (P-15) on the surface of an ABM scaffold (PepGen P-15) using AFM and FTIR. J Biomed Mater Res A. 2005. 74:712–721.

20. Qian JJ, Bhatnagar RS. Enhanced cell attachment to anorganic bone mineral in the presence of a synthetic peptide related to collagen. J Biomed Mater Res. 1996. 31:545–554.

21. Hanks T, Atkinson BL. Comparison of cell viability on anorganic bone matrix with or without P-15 cell binding peptide. Biomaterials. 2004. 25:4831–4836.

22. Yukna RA, Callan DP, Krauser JT, Evans GH, Aichelmann-Reidy ME, Moore K, et al. Multi-center clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects. 6-month results. J Periodontol. 1998. 69:655–663.

23. Ijiri S, Yamamuro T, Nakamura T, Kotani S, Notoya K. Effect of sterilization on bone morphogenetic protein. J Orthop Res. 1994. 12:628–636.

24. Asai S, Shimizu Y, Ooya K. Maxillary sinus augmentation model in rabbits: effect of occluded nasal ostium on new bone formation. Clin Oral Implants Res. 2002. 13:405–409.

25. Seol YJ, Park YJ, Lee SC, Kim KH, Lee JY, Kim TI, et al. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. J Biomed Mater Res A. 2006. 77:599–607.

27. Hwang CJ, Vaccaro AR, Lawrence JP, Hong J, Schellekens H, Alaoui-Ismaili MH, et al. Immunogenicity of bone morphogenetic proteins. J Neurosurg Spine. 2009. 10:443–451.

28. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009. 40:Suppl 3. S32–S38.

29. Saito A, Suzuki Y, Kitamura M, Ogata S, Yoshihara Y, Masuda S, et al. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an alpha-tricalcium phosphate scaffold. J Biomed Mater Res A. 2006. 77:700–706.
30. Suzuki Y, Tanihara M, Suzuki K, Saitou A, Sufan W, Nishimura Y. Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. J Biomed Mater Res. 2000. 50:405–409.

31. Lee CK, Koo KT, Park YJ, Lee JY, Rhee SH, Ku Y, et al. Biomimetic surface modification using synthetic oligopeptides for enhanced guided bone regeneration in beagles. J Periodontol. 2012. 83:101–110.

32. De Souza Nunes LS, De Oliveira RV, Holgado LA, Nary Filho H, Ribeiro DA, Matsumoto MA. Immunoexpression of Cbfa-1/Runx2 and VEGF in sinus lift procedures using bone substitutes in rabbits. Clin Oral Implants Res. 2010. 21:584–590.

33. Roberts EG, Breznak N. Mish CE, editor. Bone Physiology and Metabolism. Contemporary implant dentistry. 1994. Orlando: Mosby Year Book;557–598.
34. Bhatnagar RS, Qian JJ, Gough CA. The role in cell binding of a beta-bend within the triple helical region in collagen alpha 1 (I) chain: structural and biological evidence for conformational tautomerism on fiber surface. J Biomol Struct Dyn. 1997. 14:547–560.

35. Valentin AH, Weber J. Receptor technology--cell binding to P-15: a new method of regenerating bone quickly and safely-preliminary histomorphometrical and mechanical results in sinus floor augmentations. Keio J Med. 2004. 53:166–171.

36. Bhatnagar RS, QiannJJ , Wedychowska A, Dixon E, Smith N. Biomimetic habitats for cells: ordered matrix deposition and differentiation in gingival fibroblasts cultured on hydroxyapatitie coated with a collagen analogue. Cell Mater. 1999. 9:93–104.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download