Abstract
Purpose
Solar ultraviolet (UV) radiation causes inflammation and matrix metalloproteinase (MMP) overexpression and extracellular matrix depletion, leading to skin photoaging such as wrinkle formation, dryness, and sagging. Activation of MMP is influenced by various molecules such as reactive oxygen species (ROS), proinflammatory cytokines, and transient receptor potential vanilloid type (TRPV)-1, which are increased in UV-irradiated skin cells. Aralia elata (AE) ethanolic extract was reported to inhibit ROS generation caused by UVB-irradiation in keratinocytes. In this study, we investigated the photoprotective effect of AE ethanolic extract on UVB-irradiated human keratinocytes (HaCaT) and human dermal fibroblasts (HDF).
Methods
AE was freeze-dried, extracted in 70% ethanol, and concentrated. Skin cells were treated with AE extract for 24 h and then exposed to UVB (55 mJ/cm2). After 48 h of incubation, proinflammatory cytokines, MMP-1, type-1 procollagen, and TRPV-1 levels were measured by ELISA or Western blotting.
Results
Treatment with AE extract (100 µg/mL) significantly inhibited UVB-induced IL-6, IL-8, and PGE2 production in HaCaT by 25.6%, 5.3%, and 70.2%, respectively, and also inhibited elevation of MMP-1 and TRPV-1 caused by UVB irradiation by 20.0% and 41.9%, respectively (p < 0.05). In HDF, AE extract treatment significantly inhibited both elevation of MMP-1 and reduction of type-1 procollagen caused by UVB irradiation (p < 0.05). In addition, type-1 procollagen was elevated by AE extract treatment in normal HDFs (p < 0.05).
Figures and Tables
Fig. 1
Cell viability of HaCaT and HDF treated with Aralia elata (AE) extract. Cell viability of HaCaT and HDF were measured at 48 h treatment of AE extract. Results were shown as mean ± SD (n = 3). *Significantly different at p < 0.05 compared to the control
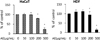
Fig. 2
Viability of HaCaT and HDF cells treated with Aralia elata (AE) extract and UVB. Cells were treated with AE extract for 24 h, exposed to UVB (55 mJ/cm2) and cell viability were measured at 48 h incubation. Results were shown as mean ± SD (n = 3). *Significantly different at p < 0.05 compared to UVB(-) control. NS: not significant

Fig. 3
Effect of Aralia elata (AE) extract on proinflammatory mediator secretion in UVB irradiated HaCaT cells. Cells were treated with AE extract for 24 h, exposed to UVB (55 mJ/cm2) and IL-6, IL-8 and PGE2 concentrations at 48 h incubation were measured in the culture medium by ELISA. Results were shown as mean ± SD (n = 3). Means sharing the same alphabet on the bar are not significantly different at p < 0.05 by ANOVA and Duncan's multiple range test.

Fig. 4
Effect of Aralia elata (AE) extract on TRPV-1 and MMP-1 protein levels in UVB-irradiated HaCaT cells. Cells were treated with AE extract for 24 h, exposed to UVB (55 mJ/cm2) and the culture medium and the cells were collected at 48 h incubation. Protein level of MMP-1 (medium), TRPV-1 and β-actin (cell) determined by western blotting (A) and quantified (B). Results were shown as mean ± SD (n = 4). Means sharing the same alphabet on the bar are not significantly different at p < 0.05 by ANOVA and Duncan's multiple range test.
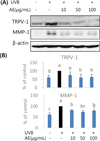
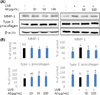
Fig. 5
Effect of Aralia elata (AE) extract on MMP-1 and type I-procollagen protein levels in normal HDFs or UVB-irradiated HDFs. Cells were treated with AE extract for 24 h, exposed to UVB (55 mJ/cm2) and the culture medium and the cells were collected at 48 h incubation. The protein level of MMP-1 (medium), type I procollagen and β-actin (cell) determined by western blotting (A) and quantified (B). Results were shown as mean ± SD (n = 3~8). Means sharing the same alphabet on the bar are not significantly different at p < 0.05 by ANOVA and Duncan's multiple range test. NS: not significant
References
1. Lee JH, Jung JH, Kim HS. Modulation of immune parameters by aging process. Korean J Nutr. 2010; 43(2):152–160.

2. Jin XJ, Kim EJ, Oh IK, Kim YK, Park CH, Chung JH. Prevention of UV-induced skin damages by 11,14,17-eicosatrienoic acid in hairless mice in vivo. J Korean Med Sci. 2010; 25(6):930–937.

3. World Health Organization. Ultraviolet radiation and the INTERSUN program: health effects of UV radiation [Internet]. Geneva: World Health Organization;cited 2016 Nov 25. Available from: http://www.who.int/uv/health/en.
4. Kwak CS, Yang J. Suppressive effects of ethanol extract of Aralia elata on UVB-induced oxidative stress in human keratinocytes. J Nutr Health. 2016; 49(3):135–143.

5. Sun Z, Park SY, Hwang E, Zhang M, Jin F, Zhang B, Yi TH. Salvianolic acid B protects normal human dermal fibroblasts against ultraviolet B irradiation-induced photoaging through mitogen-activated protein kinase and activator protein-1 pathways. Photochem Photobiol. 2015; 91(4):879–886.

6. Oh MC, Kim KC, Ko C, Ahn YS, Hyun JW. Peptides-derived from scales of Branchiostegus japonicus inhibit ultraviolet B-induced oxidative damage and photo-aging in skin cells. J Life Sci. 2015; 25(3):269–275.

8. Huang J, Qiu L, Ding L, Wang S, Wang J, Zhu Q, Song F, Hu J. Ginsenoside Rb1 and paeoniflorin inhibit transient receptor potential vanilloid-1-activated IL-8 and PGE2 production in a human keratinocyte cell line HaCaT. Int Immunopharmacol. 2010; 10(10):1279–1283.
9. Lee YM, Kang SM, Chung JH. The role of TRPV1 channel in aged human skin. J Dermatol Sci. 2012; 65(2):81–85.

10. Fernández-García E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014; 5(9):1994–2003.

11. Lee B, Kim J, Hwang J, Cho Y. Dietary effect of green tea extract on epidermal levels of skin pH related factors, lactate dehydrogenase protein expression and activity in UV-irradiated hairless mice. J Nutr Health. 2016; 49(2):63–71.

12. Hwang E, Park SY, Lee HJ, Lee TY, Sun ZW, Yi TH. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother Res. 2014; 28(12):1778–1788.

13. Lee JH, Jeong CS. Suppressive effects on the biosynthesis of inflammatory mediators by Aralia elata extract fractions in macrophage cells. Environ Toxicol Pharmacol. 2009; 28(3):333–341.

14. Cha JY, Ahn HY, Eom KE, Park BK, Jun BS, Cho YS. Antioxidative activity of Aralia elata shoot and leaf extracts. J Life Sci. 2009; 19(5):652–658.
15. Lee YJ, Lee SW, Lee SC, Park EJ. Antioxidant activities and antigenotoxic effect of ethanol extracts of Acorus gramineus, bud of Aralica elata Seem, Capsella bursa-pastoris, and Taraxacum officinale. J Basic Sci. 2014; 31:45–58.
16. Wang M, Xu X, Xu H, Wen F, Zhang X, Sun H, Yao F, Sun G, Sun X. Effect of the total saponins of Aralia elata (Miq) Seem on cardiac contractile function and intracellular calcium cycling regulation. J Ethnopharmacol. 2014; 155(1):240–247.

17. Kim SJ, Yoo WS, Kim H, Kwon JE, Hong EK, Choi M, Han Y, Chung I, Seo S, Park J, Yoo JM, Choi WS. Aralia elata prevents neuronal death by downregulating tonicity response element binding protein in diabetic retinopathy. Ophthalmic Res. 2015; 54(2):85–95.
18. Ryu MJ, Chung HS. Effect of Aralia elata on apoptosis in MDA-MB-231 human breast cancer cells. Food Eng Prog. 2015; 19(3):235–242.

19. Hwang E, Park SY, Lee HJ, Sun ZW, Lee TY, Song HG, Shin HS, Yi TH. Vigna angularis water extracts protect against ultraviolet b-exposed skin aging in vitro and in vivo. J Med Food. 2014; 17(12):1339–1349.

20. Robert L, Labat-Robert J, Robert AM. Physiology of skin aging. Pathol Biol (Paris). 2009; 57(4):336–341.

21. Kim M, Park YG, Lee HJ, Lim SJ, Nho CW. Youngiasides A and C isolated from Youngia denticulatum inhibit UVB-induced MMP expression and promote type I procollagen production via repression of MAPK/AP-1/NF-kB and activation of AMPK/Nrf2 in HaCat cells and human dermal fibroblasts. J Agric Food Chem. 2015; 63(22):5428–5438.
22. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001; 17(1):463–516.

23. Oh Y, Lim HW, Huang YH, Kwon HS, Jin CD, Kim K, Lim CJ. Attenuating properties of Agastache rugosa leaf extract against ultraviolet-B-induced photoaging via up-regulating glutathione and superoxide dismutase in a human keratinocyte cell line. J Photochem Photobiol B. 2016; 163:170–176.

24. Calò R, Marabini L. Protective effect of Vaccinium myrtillus extract against UVA- and UVB-induced damage in a human keratinocyte cell line (HaCaT cells). J Photochem Photobiol B. 2014; 132:27–35.

25. Sun S, Jiang P, Su W, Xiang Y, Li J, Zeng L, Yang S. Wild chrysanthemum extract prevents UVB radiation-induced acute cell death and photoaging. Cytotechnology. 2016; 68(2):229–240.

26. Ham SA, Hwang JS, Kang ES, Yoo T, Lim HH, Lee WJ, Paek KS, Seo HG. Ethanol extract of Dalbergia odorifera protects skin keratinocytes against ultraviolet B-induced photoaging by suppressing production of reactive oxygen species. Biosci Biotechnol Biochem. 2015; 79(5):760–766.

27. Nam JH, Seo JT, Kim YH, Kim KD, Yoo DL, Lee JN, Hong SY, Kim SJ, Sohn HB, Kim HS, Kim BS, Lee KT, Park HJ. Inhibitory effects of extracts from Smilacina japonica on lipopolysaccharide induced nitric oxide and prostaglandin E2 production in RAW264.7 macrophages. J Plant Biotechnol. 2014; 41(4):201–205.
28. Suh SJ, Jin UH, Kim KW, Son JK, Lee SH, Son KH, Chang HW, Lee YC, Kim CH. Triterpenoid saponin, oleanolic acid 3-O-beta-d-glucopyranosyl(1-->3)-alpha-l-rhamnopyranosyl(1-->2)-alphal-arabinopyranoside (OA) from Aralia elata inhibits LPS-induced nitric oxide production by down-regulated NF-κB in raw 264.7 cells. Arch Biochem Biophys. 2007; 467(2):227–233.
29. Imokawa G, Nakajima H, Ishida K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging II: over-expression of neprilysin plays an essential role. Int J Mol Sci. 2015; 16(4):7776–7795.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download