Abstract
Gastroesophageal reflux disorder (GERD) is the most common esophageal disorder in children. Achalasia occurs less commonly but has similar symptoms to GERD. A nine-year old boy presented with vomiting, heartburn, and nocturnal cough. The esophageal impedance-pH monitor revealed nonacidic GERD (all-refluxate clearance percent time of 20.9%). His symptoms persisted despite medical treatment for GERD, and he was lost to follow up. Four years later, he presented with heartburn, solid-food dysphagia, daily post-prandial vomiting, and failure to thrive. Endoscopy showed a severely dilated esophagus with candidiasis. High-resolution manometry was performed, and he was diagnosed with classic achalasia (also known as type I). His symptoms resolved after two pneumatic dilatation procedures, and his weight and height began to catch up to his peers. Clinicians might consider using high-resolution manometry in children with atypical GERD even after evaluation with an impedance-pH monitor.
Gastroesophageal reflux disorder (GERD) is the most common esophageal motility disorder in children of all ages [1]. Having occasional episodes of reflux is physiologic, whereas pathologic GERD in children consists of frequent or persistent episodes leading to esophagitis and other esophageal and respiratory symptoms [1,2]. Multichannel intraluminal impedance-pH (MII-pH) monitoring has become the gold standard of diagnosis for GERD [3]. GERD should be diagnosed with caution, particularly in children with atypical clinical presentation, even after MII-pH monitoring, because GERD might not be the primary disease [4,5].
Achalasia is a neurodegenerative disorder of the lower esophageal sphincter (LES) which occurs less commonly in children as compared to adults, and achalasia can present as progressive dysphagia, vomiting, and weight loss [6]. Esophageal manometry is the gold standard in diagnosing achalasia, but it is not convenient to perform in children. Recently, the development of high-resolution manometry (HRM) is leading to the replacement of conventional manometry. However, the experience with using HRM in children is limited.
We report a case that illustrates the pitfalls of diagnosing GERD and the value of HRM in a pediatric patient with achalasia.
A nine-year-old boy presented with a one-year history of heartburn, persistent post-prandial vomiting, infrequent dysphasia, and a nocturnal cough that developed three months prior to presentation. At another hospital, he had an upper gastrointestinal endoscopic examination and was diagnosed with acute gastritis; he was also prescribed a medication for the acute gastritis, but his symptoms did not improve. He had no history of allergies, and his family history was unremarkable.
His weight was 23.7 kg (5-10th percentile), body mass index was 14.7 (<3rd percentile), and height was 127 cm (10-25th percentile). He was afebrile, had a nocturnal cough, and had normal breath sounds on auscultation.
Laboratory results revealed a white blood cell count of 4,900/mm3 (polymorphonuclear neutrophils 20.5%, lymphocyte 64.5%, monocyte 12.0%, eosinophil 1.5%), hemoglobin of 11.7 g/dL, platelet count of 147,000/mm3. Other results are within normal limits and total IgE was slightly elevated at 78.1 IU/mL. Chest x-ray and abdominal sonography did not reveal any abnormalities.
Upper gastrointestinal endoscopy showed multiple whitish specks along the entire length of the esophagus, linear hyperemia on the body of the stomach, and normal-appearing duodenal mucosa. Pathologic studies were performed on the esophageal lesion and revealed nine eosinophils per high-power field with acanthosis. The 24-hour MII-pH monitoring demonstrated an all-reflux episodes count of 228 (reference value of adults <73), all-refluxate clearance percent-time of 20.9% (adults <1.4%), and acid/nonacid refluxate clearance percent-time of 0%/20.9%. Nonacidic reflux was found on 24-hour MII-pH monitoring, and he was diagnosed with GERD. He was treated with 15 mg to 30 mg of proton pump inhibitor once daily and prokinetic agent for 12 weeks as the treatment of GERD but symptoms persisted, so leukotriene antagonist and inhaled glucocorticoid were added for suspected eosinophilic esophagitis at 8 weeks of GERD treatment. Nocturnal vomiting resolved but post-prandial vomiting and heartburn continued to persist with 2 to 3 episodes per week after 12 weeks of the treatment. There were neither abnormal finding nor eosinophilic infiltration in follow-up upper gastrointestinal endoscopy and then he was lost to follow-up.
Four years later, he presented to our clinic with post-prandial and nocturnal heartburn and solid-food dysphagia and vomiting three times a day. At presentation, his weight was 28 kg (<3rd percentile), body mass index was 14.9 (<3rd percentile), and height was 137 cm (<3rd percentile).
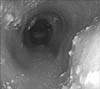
After an overnight fast, the upper gastrointestinal endoscopy revealed retention of large amounts of undigested food and secretion in the esophagus, but the stomach was empty of any food materials or fluid. After evacuating the esophagus, a dilated esophageal body and multiple whitish plaques were seen on the upper and mid-esophagus. No mucosal breaks were noted in the LES. Endoscopic biopsy of the esophagus revealed candidiasis without eosinophils (Fig. 1).
Esophagogram demonstrated delayed passage at the gastroesophageal junction (GEJ). The esophageal body was dilated but a bird-beak appearance in the distal esophagus was not obvious.
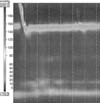
High-resolution esophageal manometric measurement of the upper esophageal sphincter pressure was within normal limits but the mean integrated relaxation pressure was 23.9 mmHg (1.5 times greater than normal value). Esophageal peristalsis was not seen on any of the swallows performed and GEJ outflow obstruction was noted (Fig. 2).
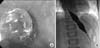
The diagnosis of type I achalasia accompanied with esophageal candidiasis was established. Esophageal candidiasis was improved after treated with fluconazole for 3 weeks and endoscopic pneumatic dilatation with balloon of 18 mm in diameter was performed (Fig. 3A). Subsequently, he gained 3 kg over a month and continued to vomit, but at a reduced rate of 2 to 3 times a week, so he received a balloon dilatation with a 24 mm diameter balloon (Fig. 3B). After the procedure, the GEJ diameter increased to 8.5 mm, and all of his symptoms resolved. The patient had a rapid recovery with a weight gain of 2 kg over the next one month. He remained completely asymptomatic for seven months after the second balloon dilatation with a total weight gain of 9 kg over nine months and a growth velocity of 6 cm/year compared with 2 cm/year prior to the balloon dilations.
GERD is a condition that develops when the reflux of stomach contents causes troublesome symptoms or complications associated with gastroesophageal reflux (GER) and is the most common esophageal disorder in children [1]. GERD has esophageal symptoms, including vomiting, dysphagia, heartburn, and poor weight gain, and extraesophageal symptoms, including cough and hoarseness.
MII detects the movement of both acidic and nonacidic fluids, solids, and air in the esophagus. Impedance is very sensitive to catheter movement and small volumes of intraluminal liquid or gas, so rapid increases in impedance may be due to gas movement or to catheter displacement in esophagus. For these reasons, volume of refluxate cannot be quantified using impedance monitoring [7]. MII-pH monitoring is a new technique that can detect temporal relationships between symptoms of GERD and the reflux of both acid and nonacid gastric contents. It showed higher rates of sensitivity and specificity than pH monitoring alone, so it had become the gold standard in the diagnosis of GERD [8].
Achalasia is an esophageal motility disorder characterized by failure of LES relaxation and is rare in children; only 5% of cases are seen before adolescence [9]. Laboratory studies indicate that achalasia is an autoimmune disease in which esophageal myenteric neurons are attacked and that achalasia is related to herpes simplex virus 1 infection in genetically predisposed subjects [10]. The most common symptoms are vomiting, dysphagia, heartburn, regurgitation, and weight loss. Childhood achalasia, especially in boys, can initially present with cough rather than the typical symptoms [6].
Achalasia can be accompanied by GER. When 48 patients with untreated achalasia were assessed for acid reflux by 24-hour ambulatory esophageal pH monitoring, about 20% of patients (10/48) experienced abnormal acid reflux, exceeding the asymptomatic control mean by 3 standard deviations [4]. GER can precede achalasia. Four of five patients reported as achalasia had GER in radiology or esophageal pH monitoring, two to five years before developing typical achalasia [5].
Anatomic damage like hiatal hernia may also cause gastroesophageal reflux in its initial phases [5]. Progressive systemic sclerosis that results in esophageal dysmotility is commonly accompanied by GERD [11]. In addition, eosinophilic esophagitis (EoE) has similar symptoms with GERD like dysphagia, heartburn, vomiting and failure to thrive [8]. So clinician might consider the possibility of these diseases in the patient not respond to treatment for GERD. But the diagnosis of EoE should be made with caution, because 34% (17/50) of the patient with achalasia had elevated intraepithelial eosinophils and 8% (4/50) met the criteria of EoE in pathologic biopsy [12]. PPI-responsive esophageal eosinophilia and other diagnosis must be excluded prior to the diagnosis of EoE [13].
Esophageal candidiasis could be seen as the consequence of aperistalsis and food stasis in achalasia. Treatment for esophageal candidiasis is recommended prior to pneumatic dilatation, because esophageal candidiasis coexisting with achalasia increase the complication risk of pneumatic dilatation, such as perforation or disseminated candidiasis [11].
Our patient met the definition of GERD showing troublesome symptoms of GERD and reflux on MII-pH monitoring, but his symptoms were caused by achalasia rather than by GERD. All of the detected reflux episodes were nonacidic. It is possible that the reflux detected in MII-pH monitoring was due to the fluctuation of retained undigested food in the dilated esophagus resulting from outflow obstruction of achalasia rather than true GERD. His symptoms were resolved after pneumatic dilatations.
It is difficult to distinguish achalasia from other diseases by symptoms alone, especially when a patient is in the early portion of the disease process or presenting with atypical symptoms. Esophagogram and esophageal manometry are essential for a definitive diagnosis of achalasia.
HRM is an advanced technology that is overcoming the limitations of conventional manometry. HRM involves a vastly increased number of pressure sensors on the manometric assembly that records detailed intraluminal pressure along the entire length of the esophagus and distinguishes between intraluminal pressurization caused by spastic contraction and trapped bolus movement in a dysfunctional esophagus. Moreover, HRM is easy to visually interpret because it uses a colored pressure topography plot [7]. Additionally, we can expect greater acceptance because our clinical experiences suggest that it is more comfortable than conventional manometry for pediatric patients.
In conclusion, it is difficult to make a differential diagnosis of achalasia by symptoms alone because achalasia has many overlapping characteristics with GERD, such as vomiting, chest pain, dysphasia, and weight loss [8,13,14]. Therefore, clinicians might consider not only GERD, but also achalasia in patients presenting with GERD-like symptoms [15] even after the diagnostic tests including MII/pH monitoring or endoscopy demonstrate positive results. Regular follow up and prompt reassessment is important. HRM may be helpful in patients unresponsive to therapy.
Figures and Tables
Fig. 1
Esophageal endoscopy of the upper and mid-esophagus revealed a dilated esophageal body and multiple whitish plaques caused by achalasia and candidiasis.
References
1. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006; 101:1900–1920.

2. Lightdale JR, Gremse DA. Section on Gastroenterology, Hepatology, and Nutrition. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013; 131:e1684–e1695.

3. Rosen R, Nurko S. The importance of multichannel intraluminal impedance in the evaluation of children with persistent respiratory symptoms. Am J Gastroenterol. 2004; 99:2452–2458.

4. Shoenut JP, Micflikier AB, Yaffe CS, Den Boer B, Teskey JM. Reflux in untreated achalasia patients. J Clin Gastroenterol. 1995; 20:6–11.

5. Smart HL, Mayberry JF, Atkinson M. Achalasia following gastro-oesophageal reflux. J R Soc Med. 1986; 79:71–73.

6. Franklin AL, Petrosyan M, Kane TD. Childhood achalasia: A comprehensive review of disease, diagnosis and therapeutic management. World J Gastrointest Endosc. 2014; 6:105–111.

7. Kahrilas PJ, Sifrim D. High-resolution manometry and impedance-pH/manometry: valuable tools in clinical and investigational esophagology. Gastroenterology. 2008; 135:756–769.

9. Roskies M, Zielinski D, Levesque D, Daniel SJ. Atypical presentations of achalasia in the pediatric population. J Otolaryngol Head Neck Surg. 2012; 41:E44–E46.
10. Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology. 2013; 145:954–965.

11. Ganatra JV, Bostwick HE, Medow MS, Beneck D, Berezin S. Candida esophagitis in a child with achalasia. J Pediatr Gastroenterol Nutr. 1996; 22:330–333.

12. Cools-Lartigue J, Chang SY, Mckendy K, Mayrand S, Marcus V, Fried GM, et al. Pattern of esophageal eosinophilic infiltration in patients with achalasia and response to Heller myotomy and Dor fundoplication. Dis Esophagus. 2013; 26:766–775.

13. Dellon ES. Diagnostics of eosinophilic esophagitis: clinical, endoscopic, and histologic pitfalls. Dig Dis. 2014; 32:48–53.

14. Loots CM, Benninga MA, Omari TI. Gastroesophageal reflux in pediatrics; (patho)physiology and new insights in diagnostics and treatment. Minerva Pediatr. 2012; 64:101–119.
15. Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ. International High Resolution Manometry Working Group. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012; 24:Suppl 1. 57–65.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download